kit - Environmental Health & Safety
advertisement

ILLINOIS STATE UNIVERSITY
CHEMICAL HYGIENE PLAN
Adopted 2-13-95
Revised February 2012
1
EMERGENCY NUMBERS
ISU POLICE
911
NORMAL FIRE DEPARTMENT
911
OFFICE OF ENVIRONMENTAL
HEALTH AND SAFETY
438-8325
JOHN GOODMAN –EHS
438-8297
STUDENT HEALTH SERVICES
438-8655
2
CHEMICAL HYGIENE PLAN FOR CHEMISTRY LABORATORIES
TABLE OF CONTENTS
1.0 INTRODUCTION..................................................................................................................6
1.1 Chemical Hygiene Plan............................................................................................. 6
1.2 Explanation of the Lab Standard............................................................................. 6
2.0 HAZARD RECOGNITION................................................................................................ 7
3.0 STANDARD OPERATING PROCEDURES FOR LABORATORY CHEMICALS...7
3.1 Administrative Procedures................................................................................... 7
a. Chemical Procurement................................................................................. 7
b. Prior Approval............................................................................................... 7
c. Working Alone - Unattended Operations................................................ 7
3.2 General Chemical Safety........................................................................................ 8
a. Horseplay......................................................................................................... 8
b. Personal Hygiene........................................................................................... 8
c. Housekeeping................................................................................................. 8
d. Material Transport........................................................................................ 9
e. Solvent Storage and Handling.................................................................... 9
f. Glassware and Laboratory Equipment....................................................... 9
g. Vacuum and Pressure Operations............................................................. 9
h. Sinks and Refrigerators................................................................................ 10
i. Compressed Gases.......................................................................................... 10
j. Fume Hoods.................................................................................................... 11
k. Cryogenic Liquids.......................................................................................... 11
l. Laboratory Freeze Dryers (Lyophilizers).................................................... 12
m. Autoclaves..................................................................................................... 12
n. Warning Signs and Labels........................................................................... 12
o. Centrifuges...................................................................................................... 13
3.3 Waste Disposal......................................................................................................... 13
a. Labeling Waste Containers.......................................................................... 14
b. Storing Waste.................................................................................................. 14
c. Having Waste Picked up for Disposal....................................................... 15
d. Radioactive Waste......................................................................................... 15
e. Potentially Infectious Material Waste....................................................... 16
f. Minimizing Waste......................................................................................... 18
g. Using Sink Drains and the Sewer.............................................................. 19
3.4 Special Chemical Safety.......................................................................................... 19
a. Corrosive Substances.................................................................................... 19
b. Oxidizers.......................................................................................................... 20
c. Oxygen and Moisture Sensitive Compounds.......................................... 20
d. Pyrophoric Compounds............................................................................... 22
3
e. Peroxide-Forming Compounds.................................................................. 22
f. Explosive and Shock-Sensitive Compounds........................................... 23
g. Incompatible Materials................................................................................. 24
h. Laser Installations......................................................................................... 24
i. Formaldehyde................................................................................................. 25
j. .Mercury............................................................................................................ 25
k. Radioactive Materials................................................................................... 25
3.5 General Biological Safety........................................................................................ 25
a. Universal Precautions.................................................................................. 25
b. Containers for Contaminated Material..................................................... 26
c. Work Area Restrictions................................................................................ 27
d. Biosafety Cabinet............................................................................................27
4.0 CRITERIA FOR IMPLEMENTATION OF CONTROL MEASURES....................... 27
4.1 Direct Methods of Control..................................................................................... 27
4.2 Engineering Methods of Control.......................................................................... 28
4.3 Ventilation Evaluation.......................................................................................... 28
5.0 PERSONAL PROTECTIVE EQUIPMENT....................................................................
5.1 Respiratory Protection............................................................................................
5.2 Eye and Face Protection..........................................................................................
5.3 Glove Use..................................................................................................................
5.4 Clothing.....................................................................................................................
28
28
29
29
29
6.0 INFORMATION AND TRAINING................................................................................ 29
6.1 Information............................................................................................................... 30
6.2 Training..................................................................................................................... 30
6.3 Material Safety Data Sheets.................................................................................... 30
6.4 Safety and Health References................................................................................ 31
7.0 APPROVAL FOR LABORATORY OPERATIONS.......................................................31
7.1 Extremely Hazardous Substances......................................................................... 31
7.2 Depleted Source Materials..................................................................................... 31
7.3 Procedures Creating Mixed Waste....................................................................... 32
7.4 Hazardous Procedures............................................................................................ 32
8.0 EXTREMELY HAZARDOUS SUBSTANCES................................................................ 32
8.1 Hazardous Substances............................................................................................. 32
a. Carcinogens..................................................................................................... 32
b. Reproductive Toxins..................................................................................... 33
c. Acute Toxins................................................................................................... 33
8.2 Procedures for Working with Hazardous Substances..................................... 33
4
9.0 SPILL RESPONSE..............................................................................................................
9.1 Awareness.................................................................................................................
9.2 Response Training...................................................................................................
9.3 Notification...............................................................................................................
34
34
35
35
10.0 ACCIDENT REPORTING.............................................................................................. 35
10.1 Reporting................................................................................................................. 35
10.2 Investigation........................................................................................................... 35
11.0 MEDICAL CONSULTATION & EVALUATION...................................................... 36
11.1 Medical Examination............................................................................................ 36
a. Criteria.............................................................................................................. 36
b. Information..................................................................................................... 36
11.2 Physician's Written Opinion.............................................................................. 36
12.0 RECORD KEEPING.........................................................................................................
12.1 Medical Records.....................................................................................................
12.2 Training Records....................................................................................................
12.3 Monitoring Records..............................................................................................
APPENDICIES
Appendix A - List of water reactive material
Appendix B - List of air reactive material
Appendix C - Incompatibility of Common Laboratory Chemicals
Appendix D - Flammable and Combustible Liquid Containment and Storage
Appendix E - Common Laboratory Corrosives
Appendix F - Common Peroxide Forming Chemicals
Appendix G - Shock Sensitive and Explosive Chemicals
Appendix H – Partial list of Carcinogenic Chemicals
Appendix I - PEL’s and TLV’s for Particularly Hazardous Substances
Appendix J – Laboratory audit form
Appendix K – OSHA Laboratory Standard
5
37
37
37
37
1.0 INTRODUCTION
1.1 Chemical Hygiene Plan
The purpose of this Chemical Hygiene Plan is to define work practices and procedures to help
ensure that laboratory workers and employees at Illinois State University are protected from
health hazards associated with the hazardous chemicals with which they work. The Chemical
Hygiene Plan is part of the University's compliance with the standard promulgated by OSHA
entitled "Hazardous Work in Laboratories." For simplicity, this standard will be referred to as
the Lab Standard in this document.
This Plan is organized into two main parts. The first is general in nature and addresses safetyrelated practices and policies common to all labs. It consists of Sections 1.0 through 12.0 and is
supplemented by Appendices B through M. The second part consists of information specific to
each lab. Hazardous material use and procedures unique to each particular lab are discussed
in this portion of the Plan. The lab-specific portion is incorporated into Appendix A.
1.2 Explanation of the Lab Standard
The Lab Standard defines a hazardous chemical as "a chemical for which there is statistically
significant evidence, based on at least one study conducted in accordance with established
scientific principles, that acute or chronic health effects may occur in employees who are
exposed to the chemical." In addition, the Lab Standard defines a laboratory as "a facility
where the laboratory use of relatively small quantities of hazardous chemicals are used on a
non-production basis." Finally, an employee in the Lab Standard is defined as "a person who is
assigned to work in a laboratory workplace and who may be exposed to hazardous chemicals
in the course of his or her assignments."
For the purposes of this Chemical Hygiene Plan, the employee described above will be called a
laboratory worker. An example of a laboratory worker would be a research or teaching
assistant, laboratory assistant or staff or faculty member instructing or performing research in
a laboratory. Students in the academic laboratory would not be considered laboratory workers,
although every effort should be made to meet the safety needs of the student within the spirit
of this standard.
If there is any confusion about whether a particular workplace is considered a laboratory
which utilizes hazardous chemicals or whether someone is considered a laboratory worker,
the Office of Environmental Health and Safety will, upon request, make this determination.
2.0 HAZARD RECOGNITION
All lab employees shall be knowledgeable concerning the hazards associated with the lab and
with the ongoing activities with in the lab. It is therefore important that staff be able to discern
6
the hazardous properties among different chemicals they use. To do so, staff shall have a basic
understanding of toxicological principles, hazard recognition, and acceptable exposure levels.
To assist with this understanding, a discussion of these subjects is provided in Appendix B.
3.0 STANDARD OPERATING PROCEDURES FOR LABORATORY CHEMICALS
3.1 Administrative Procedures
a. Chemical Procurement
Chemical containers shall not be accepted by staff without accompanying labels and
packaging in accordance with all appropriate regulations. All chemical shipments should be
dated and initialed by storeroom personnel when received, initials of user when distributed
and opened on date.
b. Prior Approval
The responsibility for approval of the acquisition and use of hazardous chemical agents rests
with the Chemical Hygiene Officer and with the Laboratory Supervisors for their laboratories.
In the absence of a designated Chemical Hygiene Officer the departmental chairperson is
ultimately responsible for the safe management all of the material procured by the
department. Certain materials including radioactive materials, lasers, explosives, recombinant
DNA, and other biohazards require prior internal (campus) or external approval at various
levels as discussed in Section 7.0. If there are questions concerning the need for approvals, the
Office of Environmental Health and Safety should be consulted.
c. Working Alone - Unattended Operations
When working with hazardous materials, it is advisable to have a second person present, or at
a minimum, maintain surveillance via telephone contact. No dangerous experiments should be
run unattended unless they are fail-safe. A dangerous experiment is one which will impose an
immediate threat to life, if there is a loss of water pressure, electricity or hood operation.
Those experiments which cannot be safely isolated shall not be performed unattended unless a
suitable monitor is present and functioning.
3.2 General Chemical Safety
a. Horseplay
Horseplay of any kind is strictly forbidden in the laboratories.
b. Personal Hygiene
Wash promptly if skin contact is made with any chemical, regardless of corrosivity. Use
emergency eyewash or shower when appropriate.
7
As a minimum, safety glasses will be worn in laboratories where hazardous materials are
being used at all times. Safety goggles and face shields will be used to increase the level of eye
protection as needed by the process.
Mouth pipeting is forbidden; use suction bulbs or other pipeting devices.
Opened toes shoes are prohibited in laboratories.
Shorts and skirts are prohibited unless wearing a lab coat.
Eating, drinking, and the application of cosmetics is forbidden in areas where hazardous
chemicals are used and shall be done only in well-defined designated areas. Do not store food
in the same refrigerator with chemicals, biohazards, or radioactive materials.
Latex cloves are for use inside the laboratory and must not be worn outside the laboratory.
Remove gloves before entering hallways.
c. Housekeeping
Adequate means of egress shall be maintained at all times.
Access to emergency equipment, showers, eyewashes, and exits must NOT be blocked in by
equipment, furniture, etc.
Material CANNOT be stored within 18” of the ceiling
Fire alarm strobe enunciators must unobstructed and visible throughout the room
Work areas and floors are not to be used for excessive storage. No unauthorized items shall be
stored in the corridors.
Promptly respond to all spills according to Section 9.0; properly dispose of the spilled chemical
and cleanup materials.
d. Material Transport
Glass or any other containers holding hazardous or radioactive materials shall be transported
using secondary containment. Some secondary containers are available for transport from the
stockroom. The use of atrium stairs for the transport of hazardous chemicals and waste is
strictly prohibited. Violators should be reported to departmental office. The freight elevator
shall not be used to transport any of the extremely hazardous materials referenced in
Appendix G.
8
e. Solvent Storage and Handling
Flammable and combustible liquids in moderate amounts (less than 5 gallons) may be stored
in the laboratory. Larger quantities require a flammable liquid storage cabinet and cannot
exceed 60 gallons of a class I flammable liquid or a class II combustible liquid within each
cabinet.
f. Glassware and Laboratory Equipment
All broken glassware will be immediately disposed of in an appropriately labeled and
identified rigid, puncture-resistant container, such as a metal trash can. Contaminated
glassware should be de-contaminated in an appropriate manner for the chemical or biohazard
used, but in such a manner, as to minimize harm from the glass to all present and future
handlers.
All laboratory equipment shall be used only for its intended purpose, unless appropriately
modified.
g. Vacuum and Pressure Operations
The hazards of high pressure systems arise largely from failures caused by leaks, pulsation,
vibration, and over pressure. Pressure gauges should be checked and recalibrated on a regular
basis.
Safety glasses are required at all times in the laboratories of Science Laboratory Building and
Felmley Hall. Extra precautions are necessary when working with vacuum and high pressure
devices. If explosion or implosion appears possible, face shields should be worn to protect the
face and neck of the user.
Note: Specific procedures should be developed for dealing with potential problems when
using vacuum and pressure operations. For example, if liquefied oxygen is suspected in a
vacuum line, evacuate room and seek faculty assistance.
h. Sinks and Refrigerators
Sinks:
May only be used for aqueous/non-hazardous material.
Must have a screen or appropriate cover over the drain to prevent solid material from entering
the drain.
9
Should have water added periodically to prevent desiccation drying of the drain trap resulting
in exposure to sewer gases and other organic vapors.
Should be kept clean and free of debris.
Refrigerators:
Explosion proof refrigerators are to be used for storage of flammable or unstable chemicals.
Under no circumstances should food or drink be stored in freezers, refrigerators or cold boxes
containing chemicals.
Stored chemicals and other materials must be tightly closed and labeled. Out of date chemicals
should be disposed of in accordance with Section 3.3.
i. Compressed Gases
Cylinders must be stored in well ventilated areas with their protective caps screwed on and
the cylinder secured (e.g., strapped or chained) to reduce the chance of the cylinder being
knocked over. Do not store cylinders near heat or high traffic areas. Flammable gas cylinders
should be stored separately form oxidizers. Large numbers of cylinders must be stored in
approved gas cylinder storage area.
Use appropriate hand carts to move cylinders. Cylinders must be secured to the cart during
transport with protective caps in place. Always consider cylinders as full and handle them
with corresponding care.
Cylinders should be secured at all times, during transport, storage and use. Never move any
gas cylinder while the regulator is still attached.
Always use the recommended regulator to dispense compressed gases.
j. Fume Hoods
All fume hoods must be evaluated by OEHS prior to their initial use and annually thereafter.
Make sure hood has been maintained in accordance with Section 4.3.
Make sure the exhaust blower is operating and air is entering the hood prior to starting an
experiment.
Do not place your face inside of the hood. Keep hands out as much as possible.
Keep sources of emission six (6) inches inside the hood.
10
Minimize the storage of chemicals in the hood. Clean up all spills immediately. Periodically
clean hood interior, including fluorescent bulb panel. If volatile or corrosive materials are
stored in the hood, it should be in continuous operation.
Do not use the hood for disposal. Use condensers, traps, or scrubbers. See Section 3.3 for waste
disposal information.
Do not handle toxic materials in a hood filled with equipment or chemicals.
k. Cryogenic Liquids
Loose-fitting heavy cloth or dry leather gloves should always be worn when handling
anything that comes in contact with cold liquids, cold solids and/or cold vapor. Gloves should
be loose fitting so that they can be removed quickly if liquids are spilled into them. A
potholder or other insulation should be used between the gloves and container except when
the material is in a dewar.
Keep container (dewar) vertical at all times. Do not roll the container on its side. Secure
dewars in restrainers to avoid spills.
Relief valves on dewars shall not be tampered with under any circumstances!
Matches, lighters, etc. and other sources of ignition are prohibited where liquid hydrogen and
oxygen are present. The use of smoking materials are prohibited anywhere in Science
Laboratory Building or Felmley Hall
Any frosting, ice formation, or excessive corrosion on safety valves may render the safety
valves inoperative. In the event of any of these instances, the vessel should be taken out of
service as these valves may not work, thus not allowing pressure release in the event of its
buildup.
Store dewars and liquid gas cylinders in well-ventilated storage areas when not in use or
connected to a closed system.
l. Laboratory Freeze Dryers (Lyophilizers)
Sign the log book at time of use.
In order to avoid implosion, use only appropriate lyophilizer flasks and inspect for cracks or
scratches that may cause failure. Do not substitute regular laboratory glassware for vacuum
use.
11
Locate the unit out of the traffic flow.
Empty the condensate trap regularly and change pump oil after large loads or every six
months.
m. Autoclaves
Sign the log book at time of use.
Any time the door is closed on the unit, assume it is fully pressurized.
Inspect the unit on a regular basis for closure alignment, cracks, damage or hot spots and clean
once a month. Never leave flammable materials, debris, or plastics in or near the unit.
When autoclaving potentially infectious material to render it non-infectious, refer to Appendix
C for proper procedures.
Under no circumstances, should the door of the autoclave be opened until the interior or
chamber pressure has been released.
Periodic spore checks should be performed to ensure sterilization time and temperatures have
been met.
n. Warning Signs and Labels
Warning Signs:
Laboratory areas that have special or unusual hazards should be posted with warning signs,
such as radiation, biological, fire, or optical hazards, etc.
Other signs should be posted to show the locations of safety showers, eyewash stations, exits,
and fire extinguishers.
Labels:
Waste containers should be labeled in accordance with Section 3.3(a).
Labels on containers of chemicals should contain information on the hazards associated with
use of the chemical.
Unlabeled bottles of chemicals should not be opened; effort should be made to determine the
contents of the bottle based on generators knowledge. Outdated materials should be disposed
of promptly.
12
Disposal cost of unknowns is the responsibility of the user's department.
o. Centrifuges
Sign the log book at time of use.
Each operator should be instructed on proper operating procedures prior to using a centrifuge.
Instructions should include requirements for balancing loads, using the proper rotor, use of
secondary containment and the use of accessory equipment.
Each employee who uses a centrifuge is responsible for the condition of the machine and rotor
at the end of the procedure.
3.3 Waste Disposal
We should strive to minimize or prevent waste generation. Waste minimization is an action of
both local and global significance, and staff are encouraged to share thoughts and ideas
concerning waste minimization and prevention.
Inevitably, some waste will be generated. Illinois State University is committed to managing
its wastes in a safe and efficient manner. These procedures govern the management of
hazardous and radioactive waste at the University.
Hazardous waste management is ruled by increasingly stringent and complex regulations.
Management of chemical and hazardous wastes at the University is accomplished by the
generator of the waste with the assistance of Environmental Health and Safety (EHS). EHS
will assist generators on campus to help assure that wastes are managed in accordance with
the regulations. However, the generator is ultimately responsible for assuring that waste
generated is managed in a safe and appropriate manner.
Any waste material that may, upon contact, present a hazard to one's health or surrounding
environment should be treated as a potentially hazardous waste. This includes spent or
unused chemicals, cleaning solutions, oils, etc.. If there is any doubt whether a material should
be treated as hazardous, contact EHS at 8-8325. Only aqueous/non-hazardous waste may be
disposed in the sewer or trash.
EHS will pick up properly documented and packaged wastes and will store them prior to their
final disposition. Waste is disposed of by contract and is picked up from the University
usually twice a year. The hierarchy of disposal methods used for the University's waste is
reclamation and residual destruction, high temperature incineration, chemical/physical
treatment, and secure or a landfill.
13
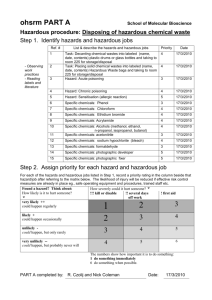
a. Labeling Waste Containers
All containers should be labeled with contents including % composition, accumulation date,
associated hazards, necessary precautions, and generator identification. Otherwise, when
container size and configuration allow, the uniform waste label shown in Figure 3.3.1 should
be used. Labels are available from EHS.
If for some reason the uniform waste label can not be used, the generator shall be sure to label
the waste container with all of the information required on the uniform label. A material safety
data sheet can often provide information necessary to label a container. MSDS's should be
obtained and kept on file for each potentially hazardous material brought on campus. A
typical MSDS is shown in Figure 3.3.2.
Only Waste labels are permitted on any waste storage containers. If the containers used for
waste are recycled from some other use then all labels must be removed and replaced with the
appropriate waste label prior to use as a waste container. When a material has not been spent
and has the original label in good condition, the original label will be sufficient.
b. Storing Waste
All waste shall be stored in a safe and secure area. Waste shall remain in such areas until
picked up by EHS. Never leave waste in a hallway or other unsecured area where it may be
subject to public contact. Wastes should be properly segregated. Halogenated materials should
be kept separate from non-halogenated and solids separated from liquids.
Generators are responsible for obtaining necessary storage containers. Containers shall be
structurally sound, in good condition, and have a tight fitting screw cap. Stoppered bottles and
plastic milk or soda bottles are not acceptable. A waste generator shall also assure that a
container is compatible with the material to be stored. Materials that may generate vapor, such
as solvents and other low boiling point materials, should be stored in a properly ventilated
area. All waste containers should have at least 10 to 20% headspace left in them to avoid
pressure build up that may result from thermal expansion.
c. Having Waste Picked Up for Disposal
Information must be provided to OEHS to adequately characterize and dispose of the waste,
prior to having it picked up. This information is provided by the generator to OEHS by using
the Pickup Request Form shown in Figure 3.3.3. Pickup requests shall be filled out and sent or
faxed to OEHS. Four to five days should be allowed for pickup.
14
OEHS will evaluate the information and if sufficient, will schedule the material for pickup. If
insufficient, OEHS will request additional information from the generator. A pickup will not
be made until appropriate information is received.
Certain wastes will require the generator to certify the presence or absence of constituents and
concentrations. This certification can be based on the generators knowledge, analytic testing,
or other scientific data. OEHS will notify generators when additional information or
certification is necessary.
The generator, defined as Laboratory Supervisor, in making the certification, accepts the
associated liability and responsibility for possible misrepresentation of the waste. Penalties
for misrepresentation, a violation of state and federal law, can include fines and/or
imprisonment.
When the generator does not have sufficient knowledge or information to make the
certification, the wastes must be analyzed at the Department's (generator's) expense. The
analysis must performed by a laboratory acceptable to OEHS and be sufficient to provide
necessary data for the generator to certify the waste. OEHS can provide guidance on
appropriate analyses.
A comprehensive analysis of an unknown waste can cost well over $1,000. It is therefore in the
generator's and Department's best interest to maintain meticulous data concerning the waste
and strict control over its composition.
d. Radioactive Waste
Radioactive waste should be stored and labeled separate from other hazardous wastes.
However, generators must assure that adequate shielding of the storage area is provided to
keep exposure as low as possible. Liquid and solid wastes should always be segregated and
collected in separate containers.
The same waste labels and request forms used for other hazardous waste should be used for
radioactive waste. The container label must indicate the chemical composition of contents,
isotopes used, quantity in microcuries, and associated hazards. This same information must
also be provided on the pickup request form.
Among hazards noted on the pickup request form should be an indication of any volatile
materials into which radioisotopes may be incorporated. That is, those that may produce
potential airborne exposures to radioisotopes.
e. Potentially Infectious Material Waste
15
Potentially Infectious Material (PIM) refers to materials or biologicals that could transfer
infectious agents to humans. The types of material are generated in connection with diagnosis,
treatment (i.e., provision of medical services), or immunization of human beings or animals;
medical research or the production or testing of biologicals. Examples of potentially infectious
materials include:
1. The following human body fluids: blood, semen, vaginal secretions, cerebrospinal fluid,
synovial fluid, pleural fluid, pericardial fluid, peritoneal fluid, amniotic fluid, saliva in dental
procedures, any body fluid that is visibly contaminated with blood, and all body fluids in
situations where it is difficult or impossible to differentiate between body fluids.
2. Any unfixed tissue, organ (other than intact skin), and body parts (except teeth and the
contiguous structures of bone and gum) from a human (living or dead).
3. HIV-containing cell or tissue cultures, organ cultures, and HIV or HBV containing culture
medium or other solutions; and blood, organs, or other tissues from experimental animals
infected with HIV or HBV.
4. Cultures and stocks of agents infectious to humans, and associated biologicals; wastes from
the production of biologicals; discarded live or attenuated vaccines; culture dishes and devices
used to transfer, inoculate, or mix cultures.
5. Waste materials originating from animals inoculated during research, production of
biologicals, or pharmaceutical testing with agents infectious to humans; carcasses, body parts,
blood, or bedding of animals known to have been in contact with agents infectious to humans.
Regulated Waste means liquid or semi-liquid blood or other potentially infectious materials
and includes the following:
1. Contaminated items that would release blood or other potentially infectious material in a
liquid or semi-liquid state if compressed
2. Items that are caked with dried blood or potentially infectious material and are capable of
releasing these materials during handling
3. Contaminated sharps and unused needles or syringes.
4. Pathological and microbiological wastes containing blood or other potentially infectious
material.
Non-Regulated Waste material includes:
16
1. Waste (except for sharps) for which the infectious potential has been eliminated by
autoclaving.
2. Sharps that meet both of the following conditions:
a. The infectious potential has been eliminated from the sharps by autoclaving.
b. The sharps are placed in leak-proof, puncture-resistant containers.
Non-regulated waste that is contained in biohazard bags or biohazard sharps containers must
first be marked "Treated" on the outside of the container, if the container does not already have
an autoclave heat/pressure tape indicator affixed to it, prior to disposing into general trash
receptacles.
Potentially infectious material can be disposed of in one of several manners.
Rendering the material non-infectious by such means as autoclaving allows the material to be
considered a non-regulated waste. See Appendix C for disposal procedures for non-regulated
waste.
Totally destroying the material through incineration requires that each department collect the
PIM in appropriate containers, store the material, and contact OEHS to pickup the material for
incineration in an EPA approved incinerator.
Under no circumstances are any sharps to be discarded into the general trash.
Departments will utilize the following storage requirements
for regulated waste prior to treatment or transport off-site.
Regulated waste must be collected or secured at the end of each day by the generators of the
waste. If there is sufficient waste in the container at the end of the day, the container should be
removed to the storage area. If the storage container is to be left in the use area, it must be
secured so no other personnel can get into the material or any of the infectious material can
contaminate any other material.
Maintain PIM in a non-putrescent state, using refrigeration when necessary.
Lock outdoor storage areas to prevent unauthorized access.
Limit access to on-site storage areas to authorized employees.
Store in a manner that affords protection from animals and does not provide a breeding place
or a food source for insects and rodents.
17
If PIM is to be rendered non-infectious by means of autoclaving the following should be
adhered to:
All autoclaving of PIM must be documented. This documentation should include the date, the
person conducting the autoclaving, the material autoclaved, and the verification that the
material was rendered non-infectious. See appendix C.
Verification that the autoclave reached the right temperature and pressure for the required
amount of time is required. One way to do this is by autoclaving, along with the waste, a jar
with spores in it. The jar is to be placed in the center of the waste bags, then if the spores are
destroyed, it is feasible that the infectious material has been rendered non-infectious. Once a
week a spore test will be required, all other times a heat/pressure tape is required to be placed
on the bags.
All autoclaves that will be used for this type of work should also be inspected prior to use with
PIMW and annually by a certified inspector. These inspections are to ensure that the
autoclaves are capable of conducting the procedures they are being used for.
f. Minimizing Waste
Waste minimization or prevention can be accomplished many different ways. Generators are
strongly encouraged to be alert for alternative procedures or products that will reduce or
prevent waste generation.
Laboratory managers should be familiar with the nature of the waste they generate, including
composition and quantity. In so doing, goals or benchmarks should be identified with efforts
focused on reaching them.
Chemicals or other materials which have not been opened or are still in usable form can be
saved from becoming waste by offering to other University staff for use.
Waste generated in both teaching and research laboratories have additional reduction options
available. These include converting to micro scale experiments and incorporating material
neutralization or inactivation into experiment procedures. This promotes environmental and
product stewardship and could be a valuable theme in course curriculum.
g. Using Sink Drains and the Sewer
Sink drains or the sewer should never be used as a means to dispose of hazardous or other
chemical waste unless it is known to environmentally compatible. Chemical and waste
products should enter the sewer only through actions incidental to the process or experiment,
18
such as container washing and rinsing. Waste material should otherwise be collected for
pickup and disposal.
Materials of questionable nature should not be put down the drain without first contacting
EHS. Never allow flammable liquids, mercury, or extremely toxic substances to enter the
sewer.
3.4 Special Chemical Safety
a. Corrosive Substances
Corrosives attack human tissue and cause irritation, chemical burns, and in severe cases, tissue
destruction. In case of skin or eye contact with corrosives, prompt treatment with a
physiologically correct buffered saline is important. Consultation with a medical professional
is required. Safety showers and eyewash fountains must be provided for this purpose and
must be readily available to all lab occupants. At no point should the storage of material or
placement of articles impede access to safety showers and eyewashes. Storage of refrigerators
and placement of chairs in alcoves where safety showers and eyewashes are present is strictly
prohibited.
In laboratories which do not have safety showers, the nearest location should be posted. All
labs should have eyewash stations.
Types of corrosives and examples of each are:
Acids:
Inorganic or mineral acids include sulfuric, nitric, hydrochloric, phosphoric and hydrofluoric.
Concentrated solutions of hydrofluoric acid (HF) can penetrate the skin and soft tissue,
causing destruction and intense pain. A neutralizing gel shall be kept in the lab any time HF is
used.
Organic acids contain a carboxylic group, (-COOH) and are generally less acidic and corrosive
than the mineral acids. Common organic acids include acetic, benzoic, citric, and oxalic.
Bases:
Bases are alkaline substances that have a pH above 7 when dissolved in water. Contact with
the skin causes a "slippery" or "soapy" feeling. Examples of common bases include:
Ammonium hydroxide
Potassium carbonate
Sodium carbonate
Calcium hydroxide
Potassium hydroxide
Sodium hydroxide
19
The eye is especially susceptible to alkalis and splash goggles or face shields are required
whenever there is a possibility of eye contact.
Halogens:
The elemental halogens (bromine, chlorine, fluorine, and iodine) are all extremely corrosive,
especially to the respiratory system. They are also capable of causing the deterioration of many
materials of construction used for gaskets, piping and tubing.
Organic Compounds:
Can be as corrosive as the inorganic acids and bases. Examples include phenols, amines and
some unsaturated ketones. In addition, many organics can be absorbed through the intact skin
and produce toxic effects.
b. Oxidizers
Oxidizers are compounds (solid, liquid, gas) that evolve oxygen or are electron acceptors
either at room temperature or upon slight heating. This group includes peroxides, chlorates,
perchlorates, nitrates, permanganates, and the elemental halogens. Oxidizers can react
vigorously at ambient temperatures when they contact organic material or reducing
substances.
c. Oxygen and Moisture Sensitive Compounds
Many chemical compounds deteriorate when exposed to air. For most of these, oxidation only
causes a decrease in purity. But for a few, extreme reactivity with oxygen leads to other effects.
Another group of compounds reacts with atmospheric moisture and causes the release of toxic
or flammable gases or vapors or the generation of enough heat to cause fires and explosions.
In the following information, the threshold limit value (TLV) is the safe amount to which a
person can be exposed to without harm.
Examples:
Compound
Effects
Aluminum Alkyls
React with moisture
extremely
flammable
vapor.
Dichlorosilane
Forms silicon dioxide and hydrogen
chloride on contact with air. Will
20
to generate
hydrocarbon
detonate spontaneously under some
conditions.
Phosphides
React with moisture to form highly toxic
phosphine (Threshold Limit ValueTLV=0.3 ppm)
Potassium
Reacts with moisture to release
hydrogen and when combined with
oxygen to cause ignition and explosion.
Selenides
Moisture causes release of the extremely
toxic hydrogen selenide (TLV=0.05
ppm)
Sodium
Reacts with moisture to release
hydrogen. The heat generated may
cause a fire.
Sulfides
Hydrogen sulfide (TLV=10 ppm)
formed on contact with moist air.
These substances should be handled in a glove box with an inert atmosphere or in special
glassware (Schlenk techniques) to avoid the aforementioned effects during experimental work.
Storage in containers with a nitrogen atmosphere is often necessary. Potassium and sodium
are usually stored under a non-volatile hydrocarbon liquid to exclude oxygen and moisture.
d. Pyrophoric Compounds
Pyrophorics are a special subgroup of air-sensitive compounds. These substances are so
reactive that they will ignite spontaneously when exposed to air. It is obvious that the
handling requirements for pyrophorics are extremely restrictive.
Examples:
Compound
Effects
Aluminum Alkyls
Ignite spontaneously in air. Also react violently with water
and with oxygenated and halogenated solvents.
21
Bromotrifluoro-
Ignites spontaneously in air to form ethylene
hydrogen bromide and hydrogen fluoride which are
corrosive and toxic.
Diborane
May ignite spontaneously in air and may detonate under
some conditions. Extremely toxic vapor (TLV=0.1 ppm)
Phosphine
Its ability to ignite spontaneously in air may depend on
purity. Phosphine gas is highly toxic (TLV=0.3 ppm)
Silane
May detonate violently when released in air, but usually it
only ignites.
The use of any of these compounds requires special approval as discussed in Section 7.0. In all
cases, a flow restrictive orifice in the cylinder valve is a required precaution. Special piping
and fittings are also necessary.
e. Peroxide-Forming Compounds
Some organic compounds are unusually susceptible to atmospheric oxidation. They require
special storage and handling procedures to minimize the formation of peroxides that may
create an explosion hazard. Once formed, peroxides are thermally unstable and may also be
shock-sensitive.
The types of organic compounds that are most apt to form peroxides include:
Aldehydes and ketones
Ethers-especially those with primary or secondary alkyl groups
Allylic or benzylic structures
Vinyl and vinylidine compounds
Avoid distilling compounds that may contain peroxides. There are test procedures for
detecting peroxide compounds and approved methods are available for destroying them once
they have formed.
Peroxide forming compounds must be dated upon receipt. Inhibited ethers can be stored for a
maximum of one year. Uninhibited ethers may only be stored for six months. After these
dates, peroxide formation may increase, thereby increasing the instability of the material.
Disposal of dated peroxide-forming materials is quite dangerous and must be accomplished
by specially trained and outfitted personnel.
22
Workers should be aware that ethers have the greatest ability to form peroxides, but the other
classes of compounds should be routinely evaluated by need and age for waste disposal.
f. Explosive and Shock-Sensitive Compounds
Shock-sensitive and/or explosive compounds are an obvious safety problem even for
laboratory-scale quantities. The first step in safe operations with such substances is a
recognition of the potential for damage and personal injury. If possible, avoid their use.
Examples:
Types
Compounds
Azides
Lead azides
Nitro-Compounds
Trinitrotoluene (TNT)
Poly-Nitrates
Nitroglycol and Nitroglycerine
Perchlorates
Perchloric acid and its salts
Picrates
Picric acid and its salts
Peroxides
Benzoyl peroxide or Methylethyl ketone peroxide
Refer to the MSDS and other literature to learn about the potential problems and the proper
procedures for working safely with these substances. Also be aware of the potential for
inadvertent formation of explosive compounds such as heavy metal perchlorates when using
perchloric acid to oxidize organic matter in an analytical procedure.
A key to safe operations with explosive or shock sensitive substances is to use very small
quantities at any one time or place.
g. Incompatible Materials
Some materials when mixed together can react violently and/or liberate toxic gas. Groups of
materials that do so are termed incompatible. Classic examples of materials that are
incompatible are cyanides or sulfides and acid. Mixing acids with either hydrogen cyanide or
hydrogen sulfide can result in the evolution of deadly gasses. Laboratory staff must be aware
of the groups of materials in their labs that could be incompatible. These materials must be
physically isolated from their incompatible counterparts. Emergency procedures must also be
in place that guides laboratory staff action in the event that materials are inadvertently mixed
23
together. A partial list of incompatible groups is given in Appendix D. Sources of information
that may help identify incompatible materials are discussed in Section 6.0.
h. Laser Installations
Lasers produce non-ionizing radiation capable of causing eye injury. Lasers operating outside
of the visible light region (ultraviolet or infrared red) are especially hazardous.
Laser dyes are complex fluorescent organic compounds. In solution with organic solvents,
these dyes form a lasing medium. Toxicity information on commercially available laser dyes is
not extensive. However, the current research has found a number of the dyes to be mutagenic
and possibly carcinogenic. The active dyes identified thus far include:
Cresyl Violet 670 Perchlorate
Coumarin 7
Coumarin 102
Coumarin 535
DCM
DODCI
LD 490
Nile Blue 690 Perchlorate
Oxazine 720 Perchlorate
p,p-Diaminoterphenyl
N,N,N'N'-Tetraethyldiaminoterphenyl
Oxazine 170 Perchlorate
Because the toxicological properties of most laser dyes have not been fully investigated, these
compounds must be handled with care.
i. Formaldehyde
OSHA has singled out formaldehyde for special regulation. This is due, in part,
to formaldehyde being implicated as a sensitizer and carcinogen. OSHA's requirement for a
formaldehyde program requires the employer to document exposure levels, provide training,
and in some cases, medical monitoring. Staff members who work with formaldehyde should
contact EHS to assure they are in compliance with the standard.
j. Mercury
Mercury and mercury compounds can be highly toxic. Mercury compounds, other than
metallic mercury, are extremely difficult to dispose of. Staff is therefore encouraged to
24
minimize mercury use and to eliminate it when possible. Mercury waste should be stored in a
non-breakable container in the fume hood.
k. Radioactive Materials
Authorized PI’s are responsible for training laboratory workers in the proper use of
radioactivity.
Proper personal protective equipment, such as lab coats, safety glasses, and disposable gloves,
must be worn when handling radioactive materials. Each person working in an area using
radioactive materials must wear a radiation film badge dosimeter unless only low betaemitters (3H and 14C) are used.
Glassware and other laboratory equipment used with radioactive materials should be rinsed
with an appropriate non-hazardous, biodegradable solvent prior to normal washing. The
solvent must be collected and stored in containers used for radioactive waste disposal, unless
the levels are <1830 dpm/gm for 3H and 14C or <50 dpm/gm for 32S and 32P. Washing of the
glassware and equipment should be done by trained personnel wearing personal protective
equipment.
All radioactive material received by staff must be properly labeled with a label bearing the
yellow-purple radiation caution symbol and the words "Caution - Radioactive Material". The
label should also include the name of the isotope, the quantity, and the date on which the last
quantity was determined.
Store all radioactive materials in locked, properly labeled cabinets or refrigerators-freezers in
properly monitored laboratories which are locked in the absence of experienced lab personnel.
3.4 General Biological Safety
a. Universal Precautions
Universal precautions shall be observed throughout all areas of Illinois State University where
reasonably anticipated skin, eye, mucous membrane, or parenteral contact with blood or other
potentially infectious material is possible.
All blood or other potentially infectious material will be considered infectious regardless of the
perceived status of the source individual and universal precautions will be taken.
Engineering and work practice controls will be utilized to eliminate or minimize exposure to
employees at the University. Where occupational exposure remains after institution of these
controls, personal protective equipment shall also be utilized.
25
b. Containers for Contaminated Material
Reusable contaminated sharps shall be placed immediately, or as soon as possible, after use
into appropriate sharps containers. These containers shall be:
i) Puncture resistant
ii) Labeled or color-coded in accordance with Appendix E
iii) Leak proof on the sides and bottom
iv) Reusable sharps that are contaminated with blood or other potentially infectious
material shall not be stored or processed in a manner that requires employees to reach
by hand into the containers where these sharps have been placed.
Reusable containers shall not be opened, emptied, or cleaned manually or in any other manner
which would expose employee to risk of percutaneous (introduced through the skin, as by
rubbing, injection, etc.) injury.
Disposable contaminated sharps shall be discarded immediately or as soon as feasible in
containers that are:
i) Closable and puncture resistant
ii) Leak proof on sides and bottom
iii) Labeled or color-coded in accordance with Appendix E
Contaminated waste other than sharps shall be placed in containers which are:
i) Closable
ii) Constructed to contain all contents and prevent leakage of fluids during handling,
storage, transport, or shipping.
iii) Labeled or color-coded in accordance with Appendix E
c. Work Area Restrictions
In work areas where there is a reasonable likelihood of exposure to blood or other potentially
infectious material, personnel are not to eat, drink, apply cosmetics or lip balm, smoke, or
handle contact lenses.
26
Food and drink shall not be kept in refrigerators, freezers, shelves, cabinets or on countertops
or benchtops where blood or other potentially infectious materials are present.
All procedures involving blood or other potentially infectious material shall be performed in
such a manner as to minimize splashing, spraying, spattering, and generation of droplets of
these substances.
d. Biosafety Cabinets
All biosafety cabinets shall be maintained according to National Sanitation Foundation
Standard 49. EHS will test and certify all biosafety cabinets annually in accordance with NSF
49.
4.0 CRITERIA FOR IMPLEMENTATION OF CONTROL MEASURES
Control measures must be implemented when exposures may be detrimental to an
individual’s health. Deciding when exposures may be detrimental will not always be easy.
However, certain circumstances will always dictate a need for control measures. These
circumstances may include an exposure above an acceptable level such as a PEL (permissible
exposure limit) or TLV, or when exposure-related health effects may be experienced by
personnel. It is very important that personnel be aware of possible symptoms of overexposure,
since some individuals may be more sensitive to a particular chemical exposure, even at levels
generally held as acceptable.
4.1 Direct Methods of Control
Direct methods of control are those which involve a change in practice concerning the use of
the toxicant. A change may involve use of a smaller amount of toxicant, alternating personnel
using the toxicant (there by reducing individual exposure), substitution with a less toxic agent,
or perhaps a change in procedure eliminating the need for the toxicant. Direct methods of
control shall always be preferred over other methods.
4.2 Engineering Methods of Control
Engineering control measures generally reduce but do not eliminate the potential for exposure.
In this sense, it can be considered an indirect method of control. The use of Personnel
protective equipment is the least preferable option to mitigate exposure. Engineering methods
of control include both local and general ventilation, equipment design and use and work area
modifications. Ventilation is of special concern as its design and operation can often be
inadequate.
27
4.3 Ventilation Evaluation
Local ventilation consists of systems designed to remove the toxicant or contaminant from the
point of generation, such as a chemical fume hood. General ventilation serves an entire work
area supplying and removing air through diffusers or vents strategically located throughout a
room. Many standards exist for proper design, maintenance, and operation of ventilation
systems.
The OSHA’s Technical Guide recommends a face velocity of 80-120 feet per minute at a sash
height of 18 inches for chemical fume hoods. Face velocity indicates the speed with which air
moves into the hood interior. Fume hoods should be used for one of two purposes; either
procedural use or storage, not both. Hood interiors should be kept free of objects that may
impede airflow. Disruption of airflow may reduce the hoods ability to protect personnel. Face
velocity is measured with an instrument called an anemometer or thermal anemometer. Face
velocity measurements are the responsibility of EHS and will be verified annually. In addition,
smoke inducing tubes should be used to verify proper airflow. Working sash height should be
as low as practical. Biological safety cabinets are the subject of specific design and operating
standards (National Sanitation Foundation Standard 49).
The Guide also recommends that air be supplied to laboratory rooms at a rate of 4 to 12 room
changes per hour. General ventilation is important in maintaining employee comfort in the
room and for removing low levels of contaminants that would be difficult to contain within a
local exhaust hood. Contact EHS if you suspect the general exhaust ventilation is insufficient.
Environmental Health and Safety can offer assistance with ventilation-related questions and
concerns.
5.0 PERSONAL PROTECTIVE EQUIPMENT
5.1 Respiratory Protection
Respirators, when properly selected and used, can offer protection against a wide variety of
airborne contaminants. However, respiratory protection should only be used when other
methods of exposure control are not effective or impractical. All respirator use must comply
with the ISU's Respiratory Protection Program. Provisions of the program include
requirements for training, regular fit testing, and medical evaluations. Copies of the program
are available upon request. EHS will perform a hazard assessment of the operation
necessitating the use of the respirator and help determine the proper level of protection
needed. EHS will also advise staff of obligations under the respiratory protection program.
5.2 Eye and Face Protection
OSHA requires that employees wear eye or face protection whenever a potential exists for
accident or injury. Any use of corrosive materials or fast moving equipment, such as
28
centrifuges, shall constitute such a potential. Face shields shall be used when potential exists
for both eye and skin injury. Goggles used shall be resistant to the types of chemicals used.
Also, if splashing or vapor penetration is possible, goggles designed for such hazards must be
used. Ultraviolet face shields should be available for use with uv lamps and transilluminators.
5.3 Glove Use
Gloves are the Personal Protective Equipment (PPE) most frequently used by laboratory staff.
Like all PPE, gloves must be properly selected for the materials to be worked with. Gloves can
be purchased in a variety of materials each offering a limited ability to resist chemical
breakthrough. Lab personnel must familiarize themselves with the limitations of the gloves
they are using and the compatibility of the glove with the chemicals likely to be encountered.
5.4 Clothing
Lab coats should be worn while performing chemical operations in which potential for
dangerous spills or splashing may occur. Similarly, during these operations, full shoes should
be worn. Shorts shall never be worn in a lab, unless covered by a lab coat or other suitable
clothing which covers the knees. Where splashing is possible, such as with large material
transfers, splash aprons should be provided. If at any time, a toxic or corrosive material
contacts clothing, the affected clothing should be immediately removed, with the affected area
rinsed under an emergency shower or sink.
6.0 INFORMATION AND TRAINING
All individuals who work in laboratories and may be exposed to hazardous chemicals must be
apprised of the hazards of chemicals present in their work area. This information and training
as outlined below must be provided before initial assignment and before new exposure
situations. Equipment necessary for the safe handling of hazardous substances must also be
provided. Environmental Health and Safety will present the basic information regarding the
Lab Standard and the information in this Plan. However, training specific for the particular lab
where an employee is assigned is the responsibility of lab director. The frequency of refresher
information and training shall be performed annually by the supervisor.
6.1 Information
Laboratory workers shall be informed of the location and availability of the following:
1. OSHA standard 1910.1450, Occupational exposure to hazardous chemicals in laboratories
2. This Chemical Hygiene Plan
29
3. Permissible exposure limits (PEL's) for OSHA regulated substances, or the recommended
exposure limits for other hazardous chemicals or threshold limit values (TLV's)
4. Signs and symptoms associated with exposure to the hazardous chemicals found in the lab
5. Material Safety Data Sheets
6.2 Training
Training shall include methods of detecting the presence of a hazardous chemical, physical
and health hazards of chemicals in the lab, and measures employees can take to protect
themselves from these hazards. The training shall present the details of the Chemical Hygiene
Plan, and shall include:
1. The contents of the OSHA Laboratory Standard and its appendices
2. The location and availability of the Chemical Hygiene Plan
3. The permissible exposure limits for OSHA regulated substances or recommended exposure
values for other hazardous chemicals not regulated by OSHA which are present in the
laboratory
4. Signs and symptoms associated with exposure to the chemicals present in the laboratory
5. Location and availability of reference material on chemical hygiene
6. The methods and observations that may be used to detect the presence or release of a
hazardous chemical
6.3 Material Safety Data Sheets (MSDS)
Chemical manufacturers and distributors must provide the purchasers of hazardous chemicals
an appropriate MSDS for each hazardous chemical purchased.
If an MSDS was not provided with the shipment of a hazardous chemical, one must be
requested in writing from the manufacturer.
Upon request, MSDS's will be made available to employees.
6.4 Safety and Health References
30
A number of resources are available from EHS for staff to review. They cover a wide variety of
topics ranging from specific chemical toxicity to general safe lab practices. Among them are:
Dangerous Properties of Industrial Materials
The Merck Index
Fundamentals of Industrial Hygiene
Laboratory Health and Safety Handbook
Sigma-Aldrich Regulatory and Safety Data
Regulatory Standards, Including Documentation of Permissible
Exposure Levels and Threshold Limit Values
Material Safety Data Sheets
7.0 APPROVAL OF LABORATORY OPERATIONS
7.1 Extremely Hazardous Materials
Procedures involving hazardous substances, such as shock sensitive -, air reactive-, or water
reactive-substances, potent carcinogens or mutagens, and/or highly virulent or pathologic
material shall not occur without prior approval from the Chemical Hygiene Officer or the
Departmental Chairperson. Any extremely hazardous substance shall not be brought onto
campus until the appropriate authorization has been received.
7.2 Depleted Source Materials
Depleted source materials such as uranium, thorium, and radium may be commonly available
in commercial products or through chemical supply companies. Because they may be naturally
occurring, some uses of depleted source materials are not regulated as other isotope use. While
obtaining these materials may be simplified, disposal is not. Disposal of source materials can
be quite difficult given the lack of available disposal alternatives. For this reason, the
acquisition of any depleted source material should be approved by OEHS and the
Departmental Chairperson using the form in Appendix L.
7.3 Procedures Creating Mixed (Hazardous and Radioactive) Waste
Mixed waste, that which possesses both hazardous and radioactive characteristics, currently
can not be disposed of. Hazardous waste facilities can not process radioactive waste and
31
radioactive waste facilities can not process hazardous wastes. Any mixed waste generated by
the University must be stored on campus indefinitely. Procedures generating mixed waste are
prohibited unless prior approval is obtained from the Chemical Hygiene Officer or the
Departmental Chairperson and OEHS using the form in Appendix L.
7.4 Hazardous Procedures
Faculty initiating new or expanded programs which include hazardous procedures and/or
usage of hazardous materials should notify the Chemical Hygiene Officer or the Departmental
Chairperson and EHS.
8.0 CLASSES OF HAZARDOUS SUBSTANCES
8.1 Hazardous Substances
OSHA’s Lab Standard requires that work with extremely hazardous chemicals be done in a
designated area. Chemicals for which special precautions are to be taken include carcinogens,
reproductive toxins and certain chemicals with a high degree of acute toxicity.
a. Carcinogens
"Select carcinogens" are defined by the Lab Standard as being any substance which meets one
of the following criteria:
1. "It is regulated by OSHA as a carcinogen; or
2. It is listed under the category, "known to be carcinogens" in the Annual Report on
Carcinogens published by the National Toxicology Program (NTP) (latest edition);
3. It is listed under Group 1 ('carcinogenic to humans') by the International Agency for
Research on Cancer Monographs(IARC)
(latest edition); or
4. It is listed in either Group 2A or 2B by IARC or under the category, reasonably anticipated
to be carcinogens by NTP, and causes statistically significant tumor incidence in experimental
animals in accordance with any of the following criteria:
a. After inhalation exposure of 6-7 hours per day, 5 days per week, for a significant
portion of a lifetime to dosages of less than 10 mg/m3;
b. After repeated skin application of less than 300 (mg/kg of body weight) per week;
or
c. After oral dosages of less than 50 mg/kg of body weight per day."
32
A list of materials which fall into these categories can be found in Appendix H.
b. Reproductive Toxins
Reproductive hazards are defined by the Lab Standard as:
"toxins (which) may manifest themselves in lethal effects on the fertilized egg,
developing embryo or fetus or teratogenic (malformation) effects in the fetus. In addition,
certain reproductive toxins may cause infertility in males and females."
Examples of reproductive toxins include: benzene, mercury, ethylene dibromide, carbon
monoxide, anesthetic gases (halothane, methoxyflurane), ionizing radiation, ethylene oxide,
ethylene thiourea, glycidyl ethers, lead and 1,2-dibromo-3-chloropropane.
c. Acute Toxins
OSHA’s Lab Standard defines "Substances with high acute toxicity such as hydrogen cyanide,
hydrogen sulfide and nitrogen dioxide are included under the category of substances for
which employers must consider the need for special precautions. Such substances may be fatal
or cause damage to target organs as a result of a single exposure or exposures of short
duration."
8.2 Procedures for Working with Hazardous Substances
Working with volatile chemicals should be performed within functioning fume hood,
ventilated glove box, sealed system, or other system designed to minimize exposure to these
substances. In all cases, work with these types of chemicals shall be done in such a manner
that the permissible exposure limits or similar standards are not exceeded.
Compressed gas cylinders which contain acutely toxic chemicals such as arsine and nitrogen
dioxide should (and may be required to) be kept in ventilated gas cabinets.
The ventilation efficiency of the designated fume hood, glove box or gas cabinets, and the
operational effectiveness of mechanical and electrical equipment used to contain or
manipulate these special substances should be evaluated periodically according to Section 4.3.
Gloves and other appropriate protective apparel must be worn.
Unless marked for "Work in Progress" (not to exceed one week) with approximate levels of
hazard, the work area shall be decontaminated upon departure from the laboratory.
33
Laboratory workers of child-bearing age should be especially cautious when working with
reproductive toxins.
9.0 SPILL RESPONSE
Chemical spills will inevitably occur in the lab and staff should be properly trained to
recognize hazards associated with the spill, mitigate the spill within their ability, and to notify
response authorities where necessary. Initial response to a spill shall always be to evacuate
the immediate area until the scope of the hazard is assessed. No staff member shall respond
to a chemical spill unless they are properly trained to do so. All lab staff must be trained to
recognize hazardous conditions associated with spills in the laboratory.
9.1 Awareness
Awareness training shall be incorporated into the initial chemical hygiene training and annual
refresher sessions. The purpose shall be to familiarize staff with hazardous materials in the
workplace, potential health effects associated with them, and response actions to be taken
when a spill is observed. Lab Supervisors are responsible for assuring that their staff has
received this training. Training shall also be provided to those that will be expected to respond
to and mitigate chemical spills.
9.2 Response Training
Chemical spills may vary in size and complexity. OSHA requires varying degrees of training
depending on an individual's response duties. Training requirements range from 8 to 40 hours.
Spills consisting of small amounts of material (a liter or less), that do not necessitate the use of
respiratory equipment to clean up, can be responded to by lab staff, provided training has
been successfully completed. Examples of minor spills that trained staff may respond to
include broken thermometers, acids, bases, and minor amounts of solvents. A wide variety of
spill response kits are available that are designed for response to minor spills. EHS can offer
guidance in kit selection. Training must also be provided to individuals that may use fire
extinguishers.
Spills involving very toxic or large quantities of materials may only be responded to by
individuals who have received formal training in emergency response. Again, such training
must comply with OSHA Standard 1910.120.
9.3 Notification
If a spill of hazardous or unknown material is observed, immediately evacuate the
surrounding area and contact EHS at 438-8325. If in the judgment of the individual the spill
may be life threatening, that individual shall immediately pull a wall station to initiate
34
building evacuation and proceed to the nearest safe location and contact ISU Police via 911,
to advise them of the situation. ISU Police will contact the appropriate response authorities.
The Chemical Hygiene Officer or the Departmental Chairperson and EHS shall be notified
when any spill is observed.
10.0 ACCIDENT REPORTING
10.1 Reporting
OSHA requires that any employee accident involving injury to be reported and recorded.
Environmental Health and Safety is responsible for recording campus accidents.
10.2 Investigation
EHS will investigate reported accidents involving injury. The purpose of the investigation will
be to determine cause and how future incidents can be prevented. Lab staff is encouraged to
participate in the investigation process.
11.0 MEDICAL CONSULTATION & EVALUATION
11.1 Medical Examination
a. Criteria
Medical examinations will be provided to all employees who work with hazardous chemicals,
including any follow-up examinations which the examining physician determines to be
necessary, under the following circumstances:
i) Employee develops signs or symptoms associated with a hazardous chemical to which the
employee may have been exposed in the laboratory.
ii) Where exposure monitoring reveals an exposure level routinely above the action level (or in
the absence of an action level, the PEL) for an OSHA regulated substance.
iii) Whenever an event takes place in the work area such as a spill, leak, explosion or other
occurrence resulting in the likelihood of a hazardous exposure.
All medical examinations and consultations shall be performed by or under the direct
supervision of a licensed physician and shall be provided without cost to the employee.
b. Information
Employer shall provide the following information to the physician:
35
1. The identity of the hazardous chemical(s) to which the employee may have been exposed.
2. A description of the conditions under which the exposure occurred including quantitative
exposure data, if available.
3. A description of the signs and symptoms of exposure that the employee is experiencing, if
any.
11.2. Physician's Written Opinion
A written opinion from the examining physician shall be obtained and shall include the
following:
1. Any recommendation for further medical follow-up.
2. The results of the medical examination and any associated tests.
3. Any medical condition which may be revealed in the course of the examination which may
place the employee at increased risk as a result of exposure to a hazardous chemical found in
the workplace.
4. A statement that the employee has been informed by the physician of the results of the
consultation or medical examination and any medical condition that may require further
examination or treatment.
The written opinion shall not reveal specific findings of diagnoses unrelated to occupational
exposure.
12.0 RECORDKEEPING
12.1 Medical Records
Medical records shall be maintained by the medical provider for at least the duration of
employment plus thirty (30) years, for each employee with occupational exposure, in
accordance with 29 CFR 1910.20.
12.2 Training Records
Training records shall include the dates of the training sessions and contents or a summary of
the training. The training records shall be maintained for three (3) years from the date on
which the training occurred.
36
12.3 Monitoring Records
EHS shall establish and maintain for each employee an accurate record of any measurements
taken to monitor employee exposure to hazardous chemicals in the laboratory.
37
APPENDIX A
Partial List of Water Reactive Chemicals:
Alkali metals, such as Na, Li, K
Alkali metal hydrides, such as LiH, CaH2, LiAlH4, NaBH4, alkali metal amides, such as
NaNH2
Metal alkyls, such as lithium and aluminum alkyls
Grignard reagents, RMgX
Halides of nonmetals, such as BCl3, BF3, PCl3, PCl5, SiCl4, S2Cl2
Inorganic acid halides, such as POCl3, SOCl2, SO2Cl2
Anhydrous metal halides, such as AlCl3, TiCl4, ZrCl4, SnCl4
Phosphorus pentoxide
Calcium carbide
Organic acid halides and anhydrides of low molecular weight, such as, acetylchloride
acetic acid nhydride
38
APPENDIX B
Partial list of Pyrophoric Chemicals
Grignard reagents, RMgX
Metal alkyls and aryls, such as RLi, RNa, R3Al, R2Zn
Metal carbonyls, such as Ni(CO)4, Fe(CO)5, Co2(CO)8
Alkali metals such as Na, K
Metal powders, such as Al, Co, Fe, Mg, Mn, Pd, Pt, Ti, Sn, Zn, Zr
Metal hydrides, such as NaH, LiAlH4
Nonmetal hydrides, such as B2H6 and other boranes, PH3, AsH3
Nonmetal alkyls, such as R3B, R3P, R3As
Phosphorus (white)
39
APPENDIX C
INCOMPATIBILITY OF COMMON LABORATORY CHEMICALS
When certain hazardous chemicals are stored or mixed together, violent reactions may occur
because the chemicals are unsuitable for mixing, or are incompatible. Classes of incompatible
chemicals should be segregated from each other during storage, according to hazard class. Use
the following general guidelines for hazard class storage:
• Flammable/Combustible Liquids and Organic Acids
• Flammable Solids
• Mineral Acids
• Caustics
• Oxidizers
• Perchloric Acid
• Compressed Gases
Before mixing any chemicals, refer to this partial list, the chemicals' MSDS's or call the EHS to
verify compatibility:
CHEMICAL INCOMPATIBLE CHEMICAL(S)
Acetic acid
aldehyde, bases, carbonates, hydroxides, metals, oxidizers,
peroxides, phosphates, xylene
Acetylene
halogens (chlorine, fluorine, etc.), mercury, potassium, oxidizers,
silver
Acetone
acids, amines, oxidizers, plastics
Alkali and alkaline earth metals acids, chromium, ethylene, halogens, hydrogen, mercury, \
nitrogen, oxidizers, plastics, sodium chloride, sulfur
Ammonia
acids, aldehydes, amides, halogens, heavy metals, oxidizers,
plastics, sulfur
Ammonium nitrate
acids, alkalis, chloride salts, combustible materials, metals, organic
materials, phosphorous, reducing agents, urea
Aniline
acids, aluminum, dibenzoyl peroxide, oxidizers, plastics
Azides
acids, heavy metals, oxidizers
Bromine
acetaldehyde, alcohol's, alkalis, amines, combustible materials,
ethylene, fluorine, hydrogen, ketones (acetone, carbonyls, etc.),
metals, sulfur
Calcium oxide
acids, ethanol, fluorine, organic materials
Carbon (activated)
alkali metals, calcium hypochlorite, halogens, oxidizers
Carbon tetrachloride
benzoyl peroxide, ethylene, fluorine, metals, oxygen, plastics,
silanes
40
Chlorates
Chromic acid
Chromium trioxide
Chlorine
Chlorine dioxide
Copper calcium
Hydroperoxide
Cyanides
Flammable liquids
Fluorine
Hydrocarbons a
powdered metals, sulfur, finely divided organic or combustible
materials
acetone, alcohol's, alkalis, ammonia, bases,
benzene, combustible materials, hydrocarbons, metals, organic
materials, phosphorous, plastics
alcohol's, ammonia, benzene, combustible materials,
Flammable compounds (hydrazine), hydrocarbons
(acetylene, ethylene, etc.),
hydrogen peroxide, iodine, metals, nitrogen, oxygen,
sodium hydroxide
hydrogen, mercury, organic materials, phosphorous, potassium
hydroxide, sulfur
hydrocarbons, oxidizers
reducing agents
acids, alkaloids, aluminum, iodine, oxidizers, strong bases
ammonium nitrate, chromic acid, hydrogen peroxide, nitric acid,
sodium peroxide, halogens
alcohol's, aldehydes, ammonia, combustible materials, halocarbons,
halogens, hydrocarbons, ketones, metals, organic acids
acids, bases, oxidizers, plastics Hydrofluoric acid metals, organic
materials, plastics, silica (glass)
CHEMICAL INCOMPATIBLE CHEMICAL(S)
Hydrogen peroxide
Hydrogen sulfide
Hypochlorites
Iodine
Mercury
Nitrates
Nitric acid
Oxalic acid
acetylaldehyde, acetic acid, acetone, alcohol's carboxylic
acid, combustible materials, metals, nitric acid, organic
compounds,
phosphorous, sulfuric acid, sodium, aniline
acetylaldehyde, metals, oxidizers, sodium
acids, activated carbon
acetylaldehyde, acetylene, ammonia, metals, sodium
acetylene, aluminum, amines, ammonia, calcium, fulminic acid,
lithium, oxidizers, sodium
acids, nitrites, metals, sulfur, sulfuric acid
acetic acid, acetonitrile, alcohol's, amines, (concentrated) ammonia,
aniline, bases, benzene, cumene, formic acid, ketones, metals,
organic materials, plastics, sodium, toluene
oxidizers, silver, sodium chlorite
41
Oxygen
acetaldehyde, secondary alcohol's, alkalis and alkalines, ammonia,
carbon monoxide, combustible materials, ethers, flammable
materials, hydrocarbons, metals, phosphorous, polymers
Perchloric acid
acetic acid, alcohol's, aniline, combustible materials, dehydrating
agents, ethyl benzene, hydriotic acid, hydrochloric acid, iodides,
ketones, organic material, oxidizers, pyridine
Peroxides, organic
acids (organic or mineral)
Phosphorus (white)
oxygen (pure and in air), alkalis
Potassium
acetylene, acids, alcohol's, halogens, hydrazine, mercury, oxidizers,
selenium, sulfur
Potassium chlorate
acids, ammonia, combustible materials, fluorine, hydrocarbons,
metals, organic materials, sugars
Potassium perchlorate
alcohol's, combustible materials, fluorine, hydrazine, metals, (also
see chlorates)organic matter, reducing agents, sulfuric acid
Potassium permanganate benzaldehyde, ethylene glycol, glycerol, sulfuric acid
Silver
acetylene, ammonia, oxidizers, ozonides, peroxyformic acid
Sodium
acids, hydrazine, metals, oxidizers, water
Sodium nitrate
acetic anhydride, acids, metals, organic matter, peroxyformic acid,
reducing agents
Sodium peroxide
acetic acid, benzene, hydrogen sulfide metals, oxidizers,
peroxyformic acid, phosphorous, reducers, sugars, water
Sulfides
acids Sulfuric acid potassium chlorates, potassium perchlorate,
potassium permanganate
References:
Michigan State University CHP taken from Material Safety Data Sheets, various chemical
companies
42
APPENDIX D
Table 10.1.1 Maximum Quantities of Flammable and Combustible Liquids and Liquefied
Flammable Gases in Sprinklered Laboratory Units of Inside Liquid Storage Areas
Excluding Quantities in
Storage Cabinets or Safety
Cans
Maximum
Quantityb
Maximum
per 9.3 m2
Quantityb per
(100 ft2 ) of
Laboratory
Laboratory
Unit
c
Unit
Laboratory
Unit Fire
Hazard Class
A
(high fire
hazard)
B
(moderate fire
hazard)
C
(low fire
hazard)
D
(minimal fire
hazard)
Including Quantities in
Storage Cabinets or Safety
Cans
Maximum
Quantityb
Maximum
per 9.3 m2
Quantityb per
(100 ft2 ) of
Laboratory
Laboratory
Unit
c
Unit
Flammable
and
Combustible
Liquid Class
Id
d
I , II, IIIA
L
gal
L
gal
L
gal
L
gal
38
76
10
20
2270
3028
600
800
76
150
20
40
4540
6060
1200
1600
Id
Id, II, IIIA
20
38
5
10
1136
1515
300
400
38
76
10
20
2270
3028
600
800
Id
I , II, IIIA
7.5
15
2
4
570
757
150
200
15
30
4
8
1136
1515
300
400
Id
Id, II, IIIA
4
4
1
1
284
284
75
75
7.5
7.5
2
2
570
570
150
150
d
Note: For maximum container sizes, see Table 10.1.4
a
See 4.2.1.3 and 11.1.6.5
b
See 4.2.2 for additional requirements for instructional and educational laboratories
c
The quantities per 9.3 m2 (100 ft2) do not imply the quantities must be within that 9.3 m2 (100 ft2) area;
the quantities per 9.3 m2 (100 ft2) are for calculation purposes to determine the total quantity.
43
APPENDIX E
COMMON LABORATORY CORROSIVES
ORGANIC ACIDS
Formic Acid
Acetic Acid (Glacial)
Propionic Acid
Butyric Acid
Chloroacetic Acid
ORGANIC BASES
Ethylenediamine
Ethylimine
Tetramethylethylenediamine
Hexamethylenediamine
Trimethylamine aq. soln.
Trichloroacetic Acid
Acetyl Chloride
Acetyl Bromide
Chloroacetyl Chloride
Oxalic Acid
Propionyl Chloride
Propionyl Bromide
Acetic Anhydride
Methyl Chloroformate
Dimethyl Sulfate
Chlorotrimethylsilane
Dichlorodimethylsilane
Phenol
Benzoyl Chloride
Benzoyl Bromide
Benzyl Chloride
Benzyl Bromide
Salicylic Acid
Triethylamine
Phenylhydrazine
Piperazine
Hydroxylamine
Tetramethylammonium
Hydroxide
ELEMENTS
Fluorine (gas)
Chlorine (gas)
Bromine (liquid)
Iodine (crystal)
Phosphorus
INORGANIC BASES
Ammonium Hydroxide
Calcium Hydroxide
Sodium Hydroxide
Potassium Hydroxide
Calcium Hydride
Sodium Hydride
Hydrazine
Ammonium Sulfide
Calcium Oxide
INORGANIC ACIDS
Hydrofluoric Acid
Hydrochloric Acid
Hydrobromic Acid
Hydriodic Acid
Sulfuric Acid
Chromerge™
No-Chromix™
Chlorosulfonic Acid
Sulfuryl Chloride
Bromine Pentafluoride
Thionyl Chloride
Tin Chloride
Tin Bromide
Titanium Tetrachloride
Perchloric Acid
Nitric Acid
Phosphoric Acid
Phosphorus Trichloride
Phosphorus Tribromide
Phosphorus
Pentachloride
Phosphorus
Pentoxide
ACID SALTS
Aluminum Trichloride
Antimony Trichloride
Ammonium Bifluoride
Calcium Fluoride
Ferric Chloride
Sodium Bisulfate
Sodium Fluoride
References :
The Foundations of Laboratory Safety, S.. R. Rayburn, 1990.
Prudent Practices for Handling Hazardous Chemicals in Laboratories,
National ResearchCouncil, 1981.
Improving Safety in the Chemical Laboratory, 2nd Ed., J. A.
Young, 1991. Material Safety Data Sheets, various chemical
companies.
44
APPENDIX F
Peroxide Forming Chemicals
Class A – Severe Peroxide Hazard
Spontaneously decompose and become explosive with exposure to air without concentration.
Butadiene (liquid monomer)
Chloroprene (liquid monomer)
Divinyl acetylene
Isopropyl ether
Potassium amide
Potassium metal
Sodium amide (sodamide)
Tetrafluoroethylene (liquid monomer)
Vinylidene chloride
Class B – Concentration Hazard
Require external energy for spontaneous decomposition. Form explosive peroxides when distilled,
evaporated or otherwise concentrated.
Acetal
Acetaldehyde
Benzyl alcohol
2-Butanol
Cumene
Cyclohexanol
Cyclohexene
2-Cyclohexen-1-ol
Decahydronaphthalene
Diacetylene
Dicyclopentadiene
Diethylene glycol dimethyl ether (diglyme)
Diethyl ether
Dioxanes
Ethylene glycol dimethyl ether (glyme)
Furan
4-Heptanol
2-Hexanol
Methylacetylene
3-Methyl-1-butanol
Methylcyclopentane
Methyl isobutyl ketone
4-Methyl-2-pentanol
2-Pentanol
4-Penten-1-ol
1-Phenylethanol
2-Phenylethanol
2-Propanol
Tetrahydrofuran
Tetrahydronaphthalene
Vinyl ethers
Other secondary alcohols
Class C – Shock and Heat Sensitive
Highly reactive and can auto-polymerize as a result of internal peroxide accumulation. The
peroxides formed in these reactions are extremely shock and heat sensitive.
Acrylic acid
Acrylonitrile
Butadiene (gas)
Chloroprene
Chlorotrifluoroethylene
Methyl methacrylate
Styrene Vinylpyridine
Tetrafluoroethylene (gas)
Vinyl acetate
Vinylacetylene (gas)
Vinyladiene chloride
Vinyl chloride (gas)
Class D – Potential Peroxide Forming Chemicals
May form peroxides but cannot be clearly categorized in Class A, B, or C.
Acrolein
Allyl ether
Allyl ethyl ether
Allyl phenyl ether
p-(n-Amyloxy)benzoyl
p-Chlorophenetole
Cyclooctene
Cyclopropyl methyl ether
Diallyl ether
p-Di-n-butoxybenzene
45
4,5-Hexadien-2-yn-1-ol
n-Hexyl ether
o.p-Iodophenetole
Isoamyl benzyl ether
Isoamyl ether
chloride
n-Amyl ether
Benzyl n-butyl ether
Benzyl ether
1,2-Dibenzyloxyethane
p-Dibenzyloxybenzene
1,2-Dichloroethyl ethyl
ether
Benzyl ethyl ether
2,4-Dichlorophenetole
Benzyl methyl ether
Benzyl-1-napthyl ether
1,2-Bis(2chloroethoxyl)ethane
Bis(2-ethoxyethyl)ether
Bis(2(methoxyethoxy)ethyl)
ether
Bis(2-chloroethyl) ether
Diethoxymethane
2,2-Diethoxypropane
Diethyl
ethoxymethylenemalonate
Diethyl fumarate
Bis(2-ethoxyethyl) adipate
Diethoxybenzene (m-,o-,p-)
Bis(2-methoxyethyl)
carbonate
Bis(2-methoxyethyl) ether
Bis(2-methoxyethyl)
phthalate
Bis(2-methoxymethyl)
adipate
Bis(2-n-butoxyethyl)
phthalate
Bis(2-phenoxyethyl) ether
Bis(4-chlorobutyl) ether
Bis(chloromethyl) ether
2-Bromomethyl ethyl ether
beta-Bromophenetole
o-Bromophenetole
p-Bromophenetole
3-Bromopropyl phenyl
ether
Isobutyl vinyl ether
Isophorone
b-Isopropoxypropionitrile
Isopropyl-2,4,5trichlorophenoxy acetate
n-Methylphenetole
2-Methyltetrahydrofuran
3-Methoxy-1-butyl acetate
2-Methoxyethanol
Diethyl acetal
3-Methoxyethyl acetate
Diethylketene
2-Methoxyethyl vinyl ether
Methoxy-1,3,5,7cyclooctatetraene
1,2-Diethoxyethane
b-Methoxypropionitrile
Dimethoxymethane
m-Nitrophenetole
1,1-Dimethoxyethane
1-Octene
Di(1-propynl) ether
Oxybis(2-ethyl acetate)
Di(2-propynl) ether
Oxybis(2-ethyl benzoate)
Di-n-propoxymethane
1,2-Epoxy-3isopropoxypropane
1,2-Epoxy-3phenoxypropane
p-Ethoxyacetophenone
1-(2-Ethoxyethoxy)ethyl
acetate
2-Ethoxyethyl acetate
(2-Ethoxyethyl)-a-benzoyl
benzoate
b,b-Oxydipropionitrile
1-Ethoxynaphthalene
n-Propyl isopropyl ether
46
1-Pentene
Phenoxyacetyl chloride
a-Phenoxypropionyl chloride
Phenyl-o-propyl ether
p-Phenylphenetone
n-Propyl ether
tert-Butyl methyl ether
n-Butyl phenyl ether
n-Butyl vinyl ether
Chloroacetaldehyde
diethylacetal
2-Chlorobutadiene
1-(2-Chloroethoxy)-2phenoxyethane
Chloroethylene
Chloromethyl methyl ether
beta-Chlorophenetole
o,p-Ethoxyphenyl
isocyanate
1-Ethoxy-2-propyne
3-Ethoxypropionitrile
Sodium 8-11-14eicosatetraenoate
Sodium ethoxyacetylide
Tetrahydropyran
2-Ethylacrylaldehyde oxime
Triethylene glycol diacetate
2-Ethylbutanol
Triethylene glycol dipropionate
Ethyl-b-ethoxypropionate
1,3,3-Trimethoxypropene
Ethylene glycol
monomethyl ether
2-Ethylhexanal
Ethyl vinyl ether
1,1,2,3-Tetrachloro-1,3butadiene
4-Vinyl cyclohexene
Vinylene carbonate
National Safety Council: Data Sheet I-655 Rev. 87
NFPA: NFPA 432, Code for the Storage of Organic Peroxide Formulations
Reactive Hazards Reduction, Inc. http://www.rhr-inc.com/
47
APPENDIX G
Shock Sensitive and Explosive Chemicals
Shock sensitive refers to the susceptibility of a chemical to rapidly decompose or explode
when struck, vibrated or otherwise agitated. Explosive chemicals are those chemicals
which have a higher propensity to explode under a given set of circumstances than other
chemicals (extreme heat, pressure, mixture with an incompatible chemical, etc.). The label
and MSDS will indicate if a chemical is shock sensitive or explosive . The chemicals listed
below may be shock sensitive or explode under a given number of circumstances and are
listed only as a guide to some shock sensitive or explosive chemicals. Follow these
guidelines:
• Write the date received and date opened on all containers of shock sensitive
chemicals. Some chemicals become increasingly shock sensitive with age.
• Unless an inhibitor was added by the manufacturer, closed containers of shock
sensitive materials should be discarded after 1 year.
• Wear appropriate personal protective equipment when handling shock sensitive
chemicals.
acetylene
acetylides of heavy metal
amatex
amatol
ammonal
ammonium nitrate
ammonium perchlorate
ammonium picrate
azides of heavy metals
baratol
calcium nitrate
chlorate
copper acetylide
cyanuric triazide
cyclotrimethylenetrinitrami
ne
dinitrophenol
fulminate of mercury
fulminate of silver
ethylene oxide
ethyl-tetryl
fulminating gold
fulminating mercury
fulminating platinum
fulminating silver
gelatinized
nitrocellulose
guanyl
guanyl nitrsamino
guanyltetrazene
hydrazine
nitrated carbohydrate
nitrated glucoside
nitrogen triiodide
Dinitrophenyl hydrazine
nitroglycerin
erythritol tetranitrate
nitrogen trichloride
ednatol
Mixtures:
germanium
hexanitrodiphenyamine
hexanitrostilbene
hexogen
hydrazoic acid
lead azide
lead mononitroresorcinate
tetracene
tetrytol
trimethylolethane
trimonite
trinitroanisole
trinitrobenzene
trinitrobenzoic acid
48
nitroguanidine
nitroparaffins
nitrourea
organic nitramines
ozonides
pentolite
perchlorates of heavy
metals
peroxides
picramic acid
picramide
picratol
picric acid
picryl sulphonic acid
silver acetylide
silver azide
tetranitromethane
dinitrotoluene
nitroglycide
lead styphnate
mannitol
trinitroresorcinol
sodium picramate
tetranitrocarbazole
trinitrocresol
hexanitrate
tritonal
urea nitrate
References: Material Safety Data Sheets, various chemical companies. Taken from MSU CHP
49
APPENDIX H
Known human carcinogens
International Agency for Research on Cancer
Group 1: Carcinogenic to humans
Acetaldehyde (from consuming alcoholic beverages)
Acid mists, strong inorganic
Aflatoxins
Alcoholic beverages
Aluminum production
4-Aminobiphenyl
Areca nut
Aristolochic acid (and plants containing it)
Arsenic and inorganic arsenic compounds
Asbestos (all forms) and mineral substances (such as talc or vermiculite) that contain
asbestos
Auramine production
Azathioprine
Benzene
Benzidine and dyes metabolized to benzidine
Benzo[a]pyrene
Beryllium and beryllium compounds
Betel quid, with or without tobacco
Bis(chloromethyl)ether and chloromethyl methyl ether (technical-grade)
Busulfan
1,3-Butadiene
Cadmium and cadmium compounds
Chlorambucil
Chlornaphazine
Chromium (VI) compounds
Clonorchis sinensis (infection with)
Coal, indoor emissions from household combustion
Coal gasification
Coal-tar distillation
Coal-tar pitch
Coke production
Cyclophosphamide
Cyclosporine
Diethylstilbestrol
50
Epstein-Barr virus (infection with)
Erionite
Estrogen postmenopausal therapy
Estrogen-progestogen postmenopausal therapy (combined)
Estrogen-progestogen oral contraceptives (combined) (Note: There is also convincing
evidence in humans that these agents confer a protective effect against cancer in the
endometrium and ovary)
Ethanol in alcoholic beverages
Ethylene oxide
Etoposide
Etoposide in combination with cisplatin and bleomycin
Fission products, including strontium-90
Formaldehyde
Haematite mining (underground)
Helicobacter pylori (infection with)
Hepatitis B virus (chronic infection with)
Hepatitis C virus (chronic infection with)
Human immunodeficiency virus type 1 (HIV-1) (infection with)
Human papilloma virus (HPV) types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 (infection
with) (Note: The HPV types that have been classified as carcinogenic to humans can
differ by an order of magnitude in risk for cervical cancer)
Human T-cell lymphotropic virus type I (HTLV-1) (infection with)
Ionizing radiation (all types)
Iron and steel founding (workplace exposure)
Isopropyl alcohol manufacture using strong acids
Kaposi sarcoma herpesvirus (KSHV)/human herpesvirus 8 (HHV-8) (infection with)
Leather dust
Magenta production
Melphalan
Methoxsalen (8-methoxypsoralen) plus ultraviolet A radiation
4,4'-Methylenebis(chloroaniline) (MOCA)
Mineral oils, untreated or mildly treated
MOPP and other combined chemotherapy including alkylating agents
2-Naphthylamine
Neutron radiation
Nickel compounds
N'-Nitrosonornicotine (NNN) and 4-(N-Nitrosomethylamino)-1-(3-pyridyl)-1-butanone
(NNK)
Opisthorchis viverrini (liver fluke; infection with)
Painter (workplace exposure as a)
51
3,4,5,3',4'-Pentachlorobiphenyl (PCB-126)
2,3,4,7,8-Pentachlorodibenzofuran
Phenacetin (and mixtures containing it)
Phosphorus-32, as phosphate
Plutonium
Radioiodines, including iodine-131
Radionuclides, alpha-particle-emitting, internally deposited (Note: Specific
radionuclides for which there is sufficient evidence for carcinogenicity to humans are
also listed individually as Group 1 agents)
Radionuclides, beta-particle-emitting, internally deposited (Note: Specific radionuclides
for which there is sufficient evidence for carcinogenicity to humans are also listed
individually as Group 1 agents)
Radium-224 and its decay products
Radium-226 and its decay products
Radium-228 and its decay products
Radon-222 and its decay products
Rubber manufacturing industry
Salted fish (Chinese-style)
Schistosoma haematobium (flatworm; infection with)
Semustine (methyl-CCNU)
Shale oils
Silica dust, crystalline, in the form of quartz or cristobalite
Solar radiation
Soot (as found in workplace exposure of chimney sweeps)
Sulfur mustard
Tamoxifen (Note: There is also conclusive evidence that tamoxifen reduces the risk of
contralateral breast cancer in breast cancer patients)
2,3,7,8-Tetrachlorodibenzo-para-dioxin
Thiotepa
Thorium-232 and its decay products
Tobacco, smokeless
Tobacco smoke, secondhand
Tobacco smoking
ortho-Toluidine
Treosulfan
Ultraviolet (UV) radiation, including UVA, UVB, and UVC rays
Ultraviolet-emitting tanning devices
Vinyl chloride
Wood dust
X- and Gamma-radiation
52
National Toxicology Program 12th Report on Carcinogens
"Known to be human carcinogens"
Aflatoxins
Alcoholic beverage consumption
4-Aminobiphenyl
Analgesic mixtures containing phenacetin
Aristolochic acids
Arsenic compounds, inorganic
Asbestos
Azathioprine
Benzene
Benzidine
Beryllium and beryllium compounds
1,3-Butadiene
1,4-Butanediol dimethylsulfonate (busulfan, Myleran®)
Cadmium and cadmium compounds
Chlorambucil
1-(2-Chloroethyl)-3-(4-methylcyclohexyl)-1-nitrosourea (MeCCNU)
bis(chloromethyl) ether and technical-grade chloromethyl methyl ether
Chromium hexavalent compounds
Coal tar pitches
Coal tars
Coke oven emissions
Cyclophosphamide
Cyclosporin A
Diethylstilbestrol (DES)
Dyes metabolized to benzidine
Environmental tobacco smoke
Erionite
Estrogens, steroidal
Ethylene oxide
Formaldehyde
Hepatitis B virus
Hepatitis C virus
Human papilloma viruses: some genital-mucosal types
Melphalan
Methoxsalen with ultraviolet A therapy (PUVA)
Mineral oils (untreated and mildly treated)
Mustard gas
53
2-Naphthylamine
Neutrons
Nickel compounds
Oral tobacco products
Radon
Silica, crystalline (respirable size)
Solar radiation
Soots
Strong inorganic acid mists containing sulfuric acid
Sunlamps or sunbeds, exposure to
Tamoxifen
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD); "dioxin"
Thiotepa
Thorium dioxide
Tobacco smoking
Vinyl chloride
Ultraviolet radiation, broad spectrum UV radiation
Wood dust
X-radiation and gamma radiation
Probable carcinogens
International Agency for Research on Cancer
Group 2A: Probably carcinogenic to humans
Acrylamide
Adriamycin (doxorubicin)
Androgenic (anabolic) steroids
Art glass, glass containers, and press ware (manufacture of)
Azacitidine
Biomass fuel (primarily wood), emissions from household combustion
Bischloroethyl nitrosourea (BCNU)
Captafol
Carbon electrode manufacture
Chloramphenicol
alpha-Chlorinated toluenes (benzal chloride, benzotrichloride, benzyl chloride) and
benzoyl chloride (combined exposures)
1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU)
4-Chloro-ortho-toluidine
54
Chlorozotocin
Cisplatin
Cobalt metal with tungsten carbide
Creosotes
Cyclopenta[cd]pyrene
Dibenz[a,h]anthracene
Dibenzo[a,l]pyrene
Diethyl sulfate
Dimethylcarbamoyl chloride
1,2-Dimethylhydrazine
Dimethyl sulfate
Engine exhaust, diesel
Epichlorohydrin
Ethyl carbamate (urethane)
Ethylene dibromide
N-Ethyl-N-nitrosourea
Frying, emissions from high-temperature
Glycidol
Hairdresser or barber (workplace exposure as)
Human papillomavirus (HPV) type 68 (infection with)
Indium phosphide
IQ (2-Amino-3-methylimidazo[4,5-f]quinoline)
Lead compounds, inorganic
Mate, hot
5-Methoxypsoralen
Methyl methanesulfonate
N-Methyl-N´-nitro-N-nitrosoguanidine (MNNG)
N-Methyl-N-nitrosourea
Nitrate or nitrite (ingested) under conditions that result in endogenous nitrosation
Nitrogen mustard
N-Nitrosodiethylamine
N-Nitrosodimethylamine
2-Nitrotoluene
Non-arsenical insecticides (workplace exposures in spraying and application of)
Petroleum refining (workplace exposures in)
Polychlorinated biphenyls (PCBs)
Procarbazine hydrochloride
Shiftwork that involves circadian disruption
Styrene-7,8-oxide
Teniposide
55
Tetrachloroethylene (perchloroethylene)
Trichloroethylene
1,2,3-Trichloropropane
Tris(2,3-dibromopropyl) phosphate
Vinyl bromide (Note: For practical purposes, vinyl bromide should be considered to act
similarly to the human carcinogen vinyl chloride.)
Vinyl fluoride (Note: For practical purposes, vinyl fluoride should be considered to act
similarly to the human carcinogen vinyl chloride.)
National Toxicology Program 12th Report on Carcinogens
"Reasonably anticipated to be human carcinogens"
Acetaldehyde
2-Acetylaminofluorene
Acrylamide
Acrylonitrile
Adriamycin® (doxorubicin hydrochloride)
2-Aminoanthraquinone
o-Aminoazotoluene
1-Amino-2,4-dibromoanthraquinone
1-Amino-2-methylanthraquinone
2-Amino-3,4-dimethylimidazo[4,5-f]quinoline (MeIQ)
2-Amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx)
2-Amino-3-methylimidazo[4,5-f]quinoline (IQ)
2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)
Amitrole
o-Anisidine hydrochloride
Azacitidine (5-Azacytidine®, 5-AzaC)
Benz[a]anthracene
Benzo[b]fluoranthene
Benzo[j]fluoranthene
Benzo[k]fluoranthene
Benzo[a]pyrene
Benzotrichloride
Bromodichloromethane
2, 2-bis-(bromoethyl)-1,3-propanediol (technical grade)
Butylated hydroxyanisole (BHA)
Captafol
Carbon tetrachloride
Ceramic fibers (respirable size)
56
Chloramphenicol
Chlorendic acid
Chlorinated paraffins (C12, 60% chlorine)
1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea
Bis(chloroethyl) nitrosourea
Chloroform
3-Chloro-2-methylpropene
4-Chloro-o-phenylenediamine
Chloroprene
p-Chloro-o-toluidine and p-chloro-o-toluidine hydrochloride
Chlorozotocin
C.I. basic red 9 monohydrochloride
Cisplatin
Cobalt sulfate
Cobalt-tungsten carbide: powders and hard metals
p-Cresidine
Cupferron
Dacarbazine
Danthron (1,8-dihydroxyanthraquinone)
2,4-Diaminoanisole sulfate
2,4-Diaminotoluene
Diazoaminobenzene
Dibenz[a,h]acridine
Dibenz[a,j]acridine
Dibenz[a,h]anthracene
7H-Dibenzo[c,g]carbazole
Dibenzo[a,e]pyrene
Dibenzo[a,h]pyrene
Dibenzo[a,i]pyrene
Dibenzo[a,l]pyrene
1,2-Dibromo-3-chloropropane
1,2-Dibromoethane (ethylene dibromide)
2,3-Dibromo-1-propanol
Tris (2,3-dibromopropyl) phosphate
1,4-Dichlorobenzene
3,3'-Dichlorobenzidine and 3,3'-dichlorobenzidine dihydrochloride
Dichlorodiphenyltrichloroethane (DDT)
1,2-Dichloroethane (ethylene dichloride)
Dichloromethane (methylene chloride)
1,3-Dichloropropene (technical grade)
57
Diepoxybutane
Diesel exhaust particulates
Diethyl sulfate
Diglycidyl resorcinol ether
3,3'-Dimethoxybenzidine
4-Dimethylaminoazobenzene
3,3'-Dimethylbenzidine
Dimethylcarbamoyl chloride
1,1-Dimethylhydrazine
Dimethyl sulfate
Dimethylvinyl chloride
1,6-Dinitropyrene
1,8-Dinitropyrene
1,4-Dioxane
Disperse blue 1
Dyes metabolized to 3,3'-dimethoxybenzidine
Dyes metabolized to 3,3'-dimethylbenzidine
Epichlorohydrin
Ethylene thiourea
Di(2-ethylhexyl) phthalate
Ethyl methanesulfonate
Furan
Glass wool fibers (inhalable)
Glycidol
Hexachlorobenzene
Hexachlorocyclohexane isomers
Hexachloroethane
Hexamethylphosphoramide
Hydrazine and hydrazine sulfate
Hydrazobenzene
Indeno[1,2,3-cd]pyrene
Iron dextran complex
Isoprene
Kepone® (chlordecone)
Lead and lead compounds
Lindane and other hexachlorocyclohexane isomers
2-Methylaziridine (propylenimine)
5-Methylchrysene
4,4'-Methylenebis(2-chloroaniline)
4-4'-Methylenebis(N,N-dimethyl)benzenamine
58
4,4'-Methylenedianiline and its dihydrochloride salt
Methyleugenol
Methyl methanesulfonate
N-methyl-N'-nitro-N-nitrosoguanidine
Metronidazole
Michler's ketone [4,4'-(dimethylamino) benzophenone]
Mirex
Naphthalene
Nickel (metallic)
Nitrilotriacetic acid
o-Nitroanisole
Nitrobenzene
6-Nitrochrysene
Nitrofen (2,4-dichlorophenyl-p-nitrophenyl ether)
Nitrogen mustard hydrochloride
Nitromethane
2-Nitropropane
1-Nitropyrene
4-Nitropyrene
N-nitrosodi-n-butylamine
N-nitrosodiethanolamine
N-nitrosodiethylamine
N-nitrosodimethylamine
N-nitrosodi-n-propylamine
N-nitroso-N-ethylurea
4-(N-nitrosomethylamino)-1-(3-pyridyl)-1-butanone
N-nitroso-N-methylurea
N-nitrosomethylvinylamine
N-nitrosomorpholine
N-nitrosonornicotine
N-nitrosopiperidine
N-nitrosopyrrolidine
N-nitrososarcosine
o-Nitrotoluene
Norethisterone
Ochratoxin A
4,4'-Oxydianiline
Oxymetholone
Phenacetin
Phenazopyridine hydrochloride
59
Phenolphthalein
Phenoxybenzamine hydrochloride
Phenytoin
Polybrominated biphenyls (PBBs)
Polychlorinated biphenyls (PCBs)
Polycyclic aromatic hydrocarbons (PAHs)
Procarbazine hydrochloride
Progesterone
1,3-Propane sultone
beta-Propiolactone
Propylene oxide
Propylthiouracil
Reserpine
Riddelliine
Safrole
Selenium sulfide
Streptozotocin
Styrene
Styrene-7,8-oxide
Sulfallate
Tetrachloroethylene (perchloroethylene)
Tetrafluoroethylene
Tetranitromethane
Thioacetamide
4,4'-Thiodianaline
Thiourea
Toluene diisocyanate
o-Toluidine and o-toluidine hydrochloride
Toxaphene
Trichloroethylene
2,4,6-Trichlorophenol
1,2,3-Trichloropropane
Ultraviolet A radiation
Ultraviolet B radiation
Ultraviolet C radiation
Urethane
Vinyl bromide
4-Vinyl-1-cyclohexene diepoxide
Vinyl fluoride
60
APPENDIX I
PEL’s and TLV’s for Particularly Hazardous Substances
The Occupational Safety and Health Administration and the American Conference of
Governmental Industrial Hygienists (ACGIH) have determined safe exposure limits for
work with hazardous chemicals. The Permissible Exposure Limits (PELs) are OSHA
standards, which must be upheld by the employer at all times. In some cases, the Threshold
Limit Value (TLV) established by ACGIH may be lower than the OSHA PEL. In these
cases, employers must strive to keep exposures as low as reasonably achievable and follow
the TLV's. Substances followed by the word skin refer to the potential for significant
adsorption through the skin. Note: PELs and TLV's are explicitly defined in the glossary
section of the appendices.
61
Laboratory Inspection Form
APPENDIX K
Room_______ Date___________ Supervisor__________________________
Area
Needs
Work
Meets
Goals
Comments
Housekeeping
floors, walls, workbenches clean
neat, clean appearance
adequate space to perform work
18 inches of ceiling clearance
Chemical Storage
proper containers compatibility
breakables not above eye level or shelf
lips/tubs in use
Chemical Container Labels
proper labels
readable labels
Hazardous Waste
central location identified
waste segregation
proper containers with readable labels
Chemical Hood
hood storage does not conflict with
usage
sash placed in proper position when
not in use
staff trained in proper use
Unsafe Conditions
broken equipment
leaking gas or water lines
unstable shelving
lack of guards on sharp edges or drive
belts
Sharps Containers
proper container in use
disposal of needles & syringes
DEPARTMENTAL ITEMS
Responsibilities of other personnel*
flushing of eyewash stations/showers
current
proper functioning of chemical hoods
recharging of fire extinguishers current
*To be checked by inspection teams. Laboratory personnel should report to the safety officer if they notice that
these items are not being done.
ADDITIONAL COMMENTS
62
APPENDIX K
• Part Number:
• Part Title:
• Subpart:
• Subpart Title:
• Standard Number:
• Title:
• Appendix:
1910
Occupational Safety and Health Standards
Z
Toxic and Hazardous Substances
1910.1450
Occupational exposure to hazardous chemicals in laboratories.
A, B
1910.1450(a)
Scope and application.
1910.1450(a)(1)
This section shall apply to all employers engaged in the laboratory use of hazardous chemicals as defined below.
1910.1450(a)(2)
Where this section applies, it shall supersede, for laboratories, the requirements of all other OSHA health standards in
29 CFR part 1910, subpart Z, except as follows:
1910.1450(a)(2)(i)
For any OSHA health standard, only the requirement to limit employee exposure to the specific permissible exposure
limit shall apply for laboratories, unless that particular standard states otherwise or unless the conditions of paragraph
(a)(2)(iii) of this section apply.
1910.1450(a)(2)(ii)
Prohibition of eye and skin contact where specified by any OSHA health standard shall be observed.
1910.1450(a)(2)(iii)
Where the action level (or in the absence of an action level, the permissible exposure limit) is routinely exceeded for an
OSHA regulated substance with exposure monitoring and medical surveillance requirements paragraphs (d) and
(g)(1)(ii) of this section shall apply.
1910.1450(a)(3)
This section shall not apply to:
1910.1450(a)(3)(i)
Uses of hazardous chemicals which do not meet the definition of laboratory use, and in such cases, the employer shall
63
comply with the relevant standard in 29 CFR part 1910, subpart Z, even if such use occurs in a laboratory.
1910.1450(a)(3)(ii)
Laboratory uses of hazardous chemicals which provide no potential for employee exposure. Examples of such
conditions might include:
1910.1450(a)(3)(ii)(A)
Procedures using chemically-impregnated test media such as Dip-and-Read tests where a reagent strip is dipped into
the specimen to be tested and the results are interpreted by comparing the color reaction to a color chart supplied by
the manufacturer of the test strip; and
1910.1450(a)(3)(ii)(B)
Commercially prepared kits such as those used in performing pregnancy tests in which all of the reagents needed to
conduct the test are contained in the kit.
1910.1450(b)
Definitions -Action level means a concentration designated in 29 CFR part 1910 for a specific substance, calculated as an eight
(8)-hour time-weighted average, which initiates certain required activities such as exposure monitoring and medical
surveillance.
Assistant Secretary means the Assistant Secretary of Labor for Occupational Safety and Health, U.S. Department of
Labor, or designee.
Carcinogen (see select carcinogen).
Chemical Hygiene Officer means an employee who is designated by the employer, and who is qualified by training
or experience, to provide technical guidance in the development and implementation of the provisions of the Chemical
Hygiene Plan. This definition is not intended to place limitations on the position description or job classification that the
designated individual shall hold within the employer's organizational structure.
Chemical Hygiene Plan means a written program developed and implemented by the employer which sets forth
procedures, equipment, personal protective equipment and work practices that (i) are capable of protecting employees
from the health hazards presented by hazardous chemicals used in that particular workplace and (ii) meets the
requirements of paragraph (e) of this section.
Combustible liquid means any liquid having a flashpoint at or above 100 deg. F (37.8 deg. C), but below 200 deg. F
(93.3 deg. C), except any mixture having components with flashpoints of 200 deg. F (93.3 deg. C), or higher, the total
volume of which make up 99 percent or more of the total volume of the mixture.
Compressed gas means:
(i) A gas or mixture of gases having, in a container, an absolute pressure exceeding 40 psi at 70 deg. F (21.1 deg. C);
or
(ii) A gas or mixture of gases having, in a container, an absolute pressure exceeding 104 psi at 130 deg. F (54.4 deg C)
regardless of the pressure at 70 deg. F (21.1 deg. C); or
(iii) A liquid having a vapor pressure exceeding 40 psi at 100 deg. F (37.8 C) as determined by ASTM D-323-72.
64
Designated area means an area which may be used for work with "select carcinogens," reproductive toxins or
substances which have a high degree of acute toxicity. A designated area may be the entire laboratory, an area of a
laboratory or a device such as a laboratory hood.
Emergency means any occurrence such as, but not limited to, equipment failure, rupture of containers or failure of
control equipment which results in an uncontrolled release of a hazardous chemical into the workplace.
Employee means an individual employed in a laboratory workplace who may be exposed to hazardous chemicals in
the course of his or her assignments.
Explosive means a chemical that causes a sudden, almost instantaneous release of pressure, gas, and heat when
subjected to sudden shock, pressure, or high temperature.
Flammable means a chemical that falls into one of the following categories:
(i) Aerosol, flammable means an aerosol that, when tested by the method described in 16 CFR 1500.45, yields a
flame protection exceeding 18 inches at full valve opening, or a flashback (a flame extending back to the valve) at any
degree of valve opening;
(ii) Gas, flammable means:
(A) A gas that, at ambient temperature and pressure, forms a flammable mixture with air at a concentration of 13
percent by volume or less; or
(B) A gas that, at ambient temperature and pressure, forms a range of flammable mixtures with air wider than 12
percent by volume, regardless of the lower limit.
(iii) Liquid, flammable means any liquid having a flashpoint below 100 deg F (37.8 deg. C), except any mixture
having components with flashpoints of 100 deg. C) or higher, the total of which make up 99 percent or more of the
total volume of the mixture.
(iv) Solid, flammable means a solid, other than a blasting agent or explosive as defined in § 1910.109(a), that is
liable to cause fire through friction, absorption of moisture, spontaneous chemical change, or retained heat from
manufacturing or processing, or which can be ignited readily and when ignited burns so vigorously and persistently as
to create a serious hazard. A chemical shall be considered to be a flammable solid if, when tested by the method
described in 16 CFR 1500.44, it ignites and burns with a self-sustained flame at a rate greater than one-tenth of an inch
per second along its major axis.
Flashpoint means the minimum temperature at which a liquid gives off a vapor in sufficient concentration to ignite
when tested as follows:
(i) Tagliabue Closed Tester (See American National Standard Method of Test for Flash Point by Tag Closed Tester,
Z11.24 - 1979 (ASTM D 56-79)) - for liquids with a viscosity of less than 45 Saybolt Universal Seconds (SUS) at 100
deg. F (37.8 deg. C), that do not contain suspended solids and do not have a tendency to form a surface film under
test; or
(ii) Pensky-Martens Closed Tester (See American National Standard Method of Test for Flashpoint by Pensky-Martens
Closed Tester, Z11.7 - 1979 (ASTM D 93-79)) - for liquids with a viscosity equal to or greater than 45 SUS at 100 deg. F
(37.8 deg. C ), or that contain suspended solids, or that have a tendency to form a surface film under test; or
(iii) Setaflash Closed Tester (see American National Standard Method of test for Flash Point by Setaflash Closed Tester
(ASTM D 3278-78)).
Organic peroxides, which undergo autoaccelerating thermal decomposition, are excluded from any of the flashpoint
determination methods specified above.
65
Hazardous chemical means a chemical for which there is statistically significant evidence based on at least one study
conducted in accordance with established scientific principles that acute or chronic health effects may occur in exposed
employees. The term "health hazard" includes chemicals which are carcinogens, toxic or highly toxic agents,
reproductive toxins, irritants, corrosives, sensitizers, hepatotoxins, nephrotoxins, neurotoxins, agents which act on the
hematopoietic systems, and agents which damage the lungs, skin, eyes, or mucous membranes.
Appendices A and B of the Hazard Communication Standard (29 CFR 1910.1200) provide further guidance in defining
the scope of health hazards and determining whether or not a chemical is to be considered hazardous for purposes of
this standard.
Laboratory means a facility where the "laboratory use of hazardous chemicals" occurs. It is a workplace where
relatively small quantities of hazardous chemicals are used on a non-production basis.
Laboratory scale means work with substances in which the containers used for reactions, transfers, and other
handling of substances are designed to be easily and safety manipulated by one person. "Laboratory scale" excludes
those workplaces whose function is to produce commercial quantities of materials.
Laboratory-type hood means a device located in a laboratory, enclosure on five sides with a movable sash or fixed
partial enclosed on the remaining side; constructed and maintained to draw air from the laboratory and to prevent or
minimize the escape of air contaminants into the laboratory; and allows chemical manipulations to be conducted in the
enclosure without insertion of any portion of the employee's body other than hands and arms.
Walk-in hoods with adjustable sashes meet the above definition provided that the sashes are adjusted during use so
that the airflow and the exhaust of air contaminants are not compromised and employees do not work inside the
enclosure during the release of airborne hazardous chemicals.
Laboratory use of hazardous chemicals means handling or use of such chemicals in which all of the following
conditions are met:
(i) Chemical manipulations are carried out on a "laboratory scale;"
(ii) Multiple chemical procedures or chemicals are used;
(iii) The procedures involved are not part of a production process, nor in any way simulate a production process; and
(iv) "Protective laboratory practices and equipment" are available and in common use to minimize the potential for
employee exposure to hazardous chemicals.
Medical consultation means a consultation which takes place between an employee and a licensed physician for the
purpose of determining what medical examinations or procedures, if any, are appropriate in cases where a significant
exposure to a hazardous chemical may have taken place.
Organic peroxide means an organic compound that contains the bivalent -O-O- structure and which may be
considered to be a structural derivative of hydrogen peroxide where one or both of the hydrogen atoms has been
replaced by an organic radical.
Oxidizer means a chemical other than a blasting agent or explosive as defined in § 1910.109(a), that initiates or
promotes combustion in other materials, thereby causing fire either of itself or through the release of oxygen or other
gases.
Physical hazard means a chemical for which there is scientifically valid evidence tat it is a combustible liquid, a
compressed gas, explosive, flammable, an organic peroxide, an oxidizer pyrophoric, unstable (reactive) or waterreactive.
Protective laboratory practices and equipment means those laboratory procedures, practices and equipment
accepted by laboratory health and safety experts as effective, or that the employer can show to be effective, in
66
minimizing the potential for employee exposure to hazardous chemicals.
Reproductive toxins means chemicals which affect the reproductive capabilities including chromosomal damage
(mutations) and effects on fetuses (teratogenesis).
Select carcinogen means any substance which meets one of the following criteria:
(i) It is regulated by OSHA as a carcinogen; or
(ii) It is listed under the category, "known to be carcinogens," in the Annual Report on Carcinogens published by the
National Toxicology Program (NTP)(latest edition); or
(iii) It is listed under Group 1 ("carcinogenic to humans") by the International Agency for research on Cancer
Monographs (IARC)(latest editions); or
(iv) It is listed in either Group 2A or 2B by IARC or under the category, "reasonably anticipated to be carcinogens" by
NTP, and causes statistically significant tumor incidence in experimental animals in accordance with any of the following
criteria:
(A) After inhalation exposure of 6-7 hours per day, 5 days per week, for a significant portion of a lifetime to dosages of
less than 10 mg/m(3);
(B) After repeated skin application of less than 300 (mg/kg of body weight) per week; or
(C) After oral dosages of less than 50 mg/kg of body weight per day.
Unstable (reactive) means a chemical which is the pure state, or as produced or transported, will vigorously
polymerize, decompose, condense, or will become self-reactive under conditions of shocks, pressure or temperature.
Water-reactive means a chemical that reacts with water to release a gas that is either flammable or presents a health
hazard.
1910.1450(c)
Permissible exposure limits. For laboratory uses of OSHA regulated substances, the employer shall assure that
laboratory employees' exposures to such substances do not exceed the permissible exposure limits specified in 29 CFR
part 1910, subpart Z.
1910.1450(d)
Employee exposure determination -1910.1450(d)(1)
Initial monitoring. The employer shall measure the employee's exposure to any substance regulated by a standard
which requires monitoring if there is reason to believe that exposure levels for that substance routinely exceed the
action level (or in the absence of an action level, the PEL).
1910.1450(d)(2)
Periodic monitoring. If the initial monitoring prescribed by paragraph (d)(1) of this section discloses employee
exposure over the action level (or in the absence of an action level, the PEL), the employer shall immediately comply
with the exposure monitoring provisions of the relevant standard.
67
1910.1450(d)(3)
Termination of monitoring. Monitoring may be terminated in accordance with the relevant standard.
1910.1450(d)(4)
Employee notification of monitoring results. The employer shall, within 15 working days after the receipt of any
monitoring results, notify the employee of these results in writing either individually or by posting results in an
appropriate location that is accessible to employees.
1910.1450(e)
Chemical hygiene plan -- General. (Appendix A of this section is non-mandatory but provides guidance to assist
employers in the development of the Chemical Hygiene Plan).
1910.1450(e)(1)
Where hazardous chemicals as defined by this standard are used in the workplace, the employer shall develop and
carry out the provisions of a written Chemical Hygiene Plan which is:
1910.1450(e)(1)(i)
Capable of protecting employees from health hazards associated with hazardous chemicals in that laboratory and
1910.1450(e)(1)(ii)
Capable of keeping exposures below the limits specified in paragraph (c) of this section.
1910.1450(e)(2)
The Chemical Hygiene Plan shall be readily available to employees, employee representatives and, upon request, to the
Assistant Secretary.
1910.1450(e)(3)
The Chemical Hygiene Plan shall include each of the following elements and shall indicate specific measures that the
employer will take to ensure laboratory employee protection;
1910.1450(e)(3)(i)
Standard operating procedures relevant to safety and health considerations to be followed when laboratory work
involves the use of hazardous chemicals;
1910.1450(e)(3)(ii)
Criteria that the employer will use to determine and implement control measures to reduce employee exposure to
hazardous chemicals including engineering controls, the use of personal protective equipment and hygiene practices;
particular attention shall be given to the selection of control measures for chemicals that are known to be extremely
hazardous;
1910.1450(e)(3)(iii)
A requirement that fume hoods and other protective equipment are functioning properly and specific measures that
68
shall be taken to ensure proper and adequate performance of such equipment;
1910.1450(e)(3)(iv)
Provisions for employee information and training as prescribed in paragraph (f) of this section;
1910.1450(e)(3)(v)
The circumstances under which a particular laboratory operation, procedure or activity shall require prior approval from
the employer or the employer's designee before implementation;
1910.1450(e)(3)(vi)
Provisions for medical consultation and medical examinations in accordance with paragraph (g) of this section;
1910.1450(e)(3)(vii)
Designation of personnel responsible for implementation of the Chemical Hygiene Plan including the assignment of a
Chemical Hygiene Officer, and, if appropriate, establishment of a Chemical Hygiene Committee; and
1910.1450(e)(3)(viii)
Provisions for additional employee protection for work with particularly hazardous substances. These include "select
carcinogens," reproductive toxins and substances which have a high degree of acute toxicity. Specific consideration
shall be given to the following provisions which shall be included where appropriate:
1910.1450(e)(3)(viii)(A)
Establishment of a designated area;
1910.1450(e)(3)(viii)(B)
Use of containment devices such as fume hoods or glove boxes;
1910.1450(e)(3)(viii)(C)
Procedures for safe removal of contaminated waste; and
1910.1450(e)(3)(viii)(D)
Decontamination procedures.
1910.1450(e)(4)
The employer shall review and evaluate the effectiveness of the Chemical Hygiene Plan at least annually and update it
as necessary.
1910.1450(f)
Employee information and training.
1910.1450(f)(1)
The employer shall provide employees with information and training to ensure that they are apprised of the hazards of
69
chemicals present in their work area.
1910.1450(f)(2)
Such information shall be provided at the time of an employee's initial assignment to a work area where hazardous
chemicals are present and prior to assignments involving new exposure situations. The frequency of refresher
information and training shall be determined by the employer.
1910.1450(f)(3)
Information. Employees shall be informed of:
1910.1450(f)(3)(i)
The contents of this standard and its appendices which shall be made available to employees;
1910.1450(f)(3)(ii)
the location and availability of the employer's Chemical Hygiene Plan;
1910.1450(f)(3)(iii)
The permissible exposure limits for OSHA regulated substances or recommended exposure limits for other hazardous
chemicals where there is no applicable OSHA standard;
1910.1450(f)(3)(iv)
Signs and symptoms associated with exposures to hazardous chemicals used in the laboratory; and
1910.1450(f)(3)(v)
The location and availability of known reference material on the hazards, safe handling, storage and disposal of
hazardous chemicals found in the laboratory including, but not limited to, Material Safety Data Sheets received from the
chemical supplier.
1910.1450(f)(4)
Training.
1910.1450(f)(4)(i)
Employee training shall include:
1910.1450(f)(4)(i)(A)
Methods and observations that may be used to detect the presence or release of a hazardous chemical (such as
monitoring conducted by the employer, continuous monitoring devices, visual appearance or odor of hazardous
chemicals when being released, etc.);
1910.1450(f)(4)(i)(B)
The physical and health hazards of chemicals in the work area; and
70
1910.1450(f)(4)(i)(C)
The measures employees can take to protect themselves from these hazards, including specific procedures the
employer has implemented to protect employees from exposure to hazardous chemicals, such as appropriate work
practices, emergency procedures, and personal protective equipment to be used.
1910.1450(f)(4)(ii)
The employee shall be trained on the applicable details of the employer's written Chemical Hygiene Plan.
1910.1450(g)
Medical consultation and medical examinations.
1910.1450(g)(1)
The employer shall provide all employees who work with hazardous chemicals an opportunity to receive medical
attention, including any follow-up examinations which the examining physician determines to be necessary, under the
following circumstances:
1910.1450(g)(1)(i)
Whenever an employee develops signs or symptoms associated with a hazardous chemical to which the employee may
have been exposed in the laboratory, the employee shall be provided an opportunity to receive an appropriate medical
examination.
1910.1450(g)(1)(ii)
Where exposure monitoring reveals an exposure level routinely above the action level (or in the absence of an action
level, the PEL) for an OSHA regulated substance for which there are exposure monitoring and medical surveillance
requirements, medical surveillance shall be established for the affected employee as prescribed by the particular
standard.
1910.1450(g)(1)(iii)
Whenever an event takes place in the work area such as a spill, leak, explosion or other occurrence resulting in the
likelihood of a hazardous exposure, the affected employee shall be provided an opportunity for a medical consultation.
Such consultation shall be for the purpose of determining the need for a medical examination.
1910.1450(g)(2)
All medical examinations and consultations shall be performed by or under the direct supervision of a licensed physician
and shall be provided without cost to the employee, without loss of pay and at a reasonable time and place.
1910.1450(g)(3)
Information provided to the physician. The employer shall provide the following information to the physician:
1910.1450(g)(3)(i)
The identity of the hazardous chemical(s) to which the employee may have been exposed;
1910.1450(g)(3)(ii)
71
A description of the conditions under which the exposure occurred including quantitative exposure data, if available;
and
1910.1450(g)(3)(iii)
A description of the signs and symptoms of exposure that the employee is experiencing, if any.
1910.1450(g)(4)
Physician's written opinion.
1910.1450(g)(4)(i)
For examination or consultation required under this standard, the employer shall obtain a written opinion from the
examining physician which shall include the following:
1910.1450(g)(4)(i)(A)
Any recommendation for further medical follow-up;
1910.1450(g)(4)(i)(B)
The results of the medical examination and any associated tests;
1910.1450(g)(4)(i)(C)
Any medical condition which may be revealed in the course of the examination which may place the employee at
increased risk as a result of exposure to a hazardous workplace; and
1910.1450(g)(4)(i)(D)
A statement that the employee has been informed by the physician of the results of the consultation or medical
examination and any medical condition that may require further examination or treatment.
1910.1450(g)(4)(ii)
The written opinion shall not reveal specific findings of diagnoses unrelated to occupational exposure.
1910.1450(h)
Hazard identification.
1910.1450(h)(1)
With respect to labels and material safety data sheets:
1910.1450(h)(1)(i)
Employers shall ensure that labels on incoming containers of hazardous chemicals are not removed or defaced.
1910.1450(h)(1)(ii)
Employers shall maintain any material safety data sheets that are received with incoming shipments of hazardous
72
chemicals, and ensure that they are readily accessible to laboratory employees.
1910.1450(h)(2)
The following provisions shall apply to chemical substances developed in the laboratory:
1910.1450(h)(2)(i)
If the composition of the chemical substance which is produced exclusively for the laboratory's use is known, the
employer shall determine if it is a hazardous chemical as defined in paragraph (b) of this section. If the chemical is
determined to be hazardous, the employer shall provide appropriate training as required under paragraph (f) of this
section.
1910.1450(h)(2)(ii)
If the chemical produced is a byproduct whose composition is not known, the employer shall assume that the
substance is hazardous and shall implement paragraph (e) of this section.
1910.1450(h)(2)(iii)
If the chemical substance is produced for another user outside of the laboratory, the employer shall comply with the
Hazard Communication Standard (29 CFR 1910.1200) including the requirements for preparation of material safety data
sheets and labeling.
1910.1450(i)
Use of respirators. Where the use of respirators is necessary to maintain exposure below permissible exposure limits,
the employer shall provide, at no cost to the employee, the proper respiratory equipment. Respirators shall be selected
and used in accordance with the requirements of 29 CFR 1910.134.
1910.1450(j)
Recordkeeping.
1910.1450(j)(1)
The employer shall establish and maintain for each employee an accurate record of any measurements taken to
monitor employee exposures and any medical consultation and examinations including tests or written opinions
required by this standard.
1910.1450(j)(2)
The employer shall assure that such records are kept, transferred, and made available in accordance with 29 CFR
1910.1020.
1910.1450(k)
[Reserved]
1910.1450(l)
Appendices. The information contained in the appendices is not intended, by itself, to create any additional
obligations not otherwise imposed or to detract from any existing obligation.
73
[55 FR 3327, Jan. 31, 1990; 55 FR 7967, March, 6, 1990; 55 FR 12777, March 30, 1990; 61 FR 5507, Feb. 13, 1996; 71
FR 16674, April 3, 2006]
• Part Number:
• Part Title:
• Subpart:
• Subpart Title:
• Standard
Number:
• Title:
1910
Occupational Safety and Health Standards
Z
Toxic and Hazardous Substances
1910.1450 App A
National Research Council Recommendations Concerning Chemical Hygiene in Laboratories (NonMandatory)
Table of Contents
Foreword
Corresponding Sections of the Standard and This Appendix
A. General Principles
1. Minimize all Chemical Exposures
2. Avoid Underestimation of Risk
3. Provide Adequate Ventilation
4. Institute a Chemical Hygiene Program
5. Observe the PELs and TLVs
B. Responsibilities
1. Chief Executive Officer
2. Supervisor of Administrative Unit
3. Chemical Hygiene Officer
4. Laboratory Supervisor
5. Project Director
6. Laboratory Worker
C. The Laboratory Facility
1. Design
2. Maintenance
74
3. Usage
4. Ventilation
D. Components of the Chemical Hygiene Plan
1. Basic Rules and Procedures
2. Chemical Procurement, Distribution, and Storage
3. Environmental Monitoring
4. Housekeeping, Maintenance and Inspections
5. Medical Program
6. Personal Protective Apparel and Equipment
7. Records
8. Signs and Labels
9. Spills and Accidents
10. Training and Information
11. Waste Disposal
E. General Procedures for Working With Chemicals
1. General Rules for all Laboratory Work with Chemicals
2. Allergens and Embryotoxins
3. Chemicals of Moderate Chronic or High Acute Toxicity
4. Chemicals of High Chronic Toxicity
5. Animal Work with Chemicals of High Chronic Toxicity
F. Safety Recommendations
G. Material Safety Data Sheets
Foreword
As guidance for each employer's development of an appropriate laboratory Chemical Hygiene Plan, the following nonmandatory recommendations are provided. They were extracted form "Prudent Practices" for Handling Hazardous
Chemicals in Laboratories" (referred to below as "Prudent Practices"), which was published in 1981 by the National
Research Council and is available from the National Academy Press, 2101 Constitution Ave., NW,. Washington DC
75
20418.
"Prudent Practices" is cited because of its wide distribution and acceptance and because of its preparation by members
of the laboratory community through the sponsorship of the National Research Council. However, none of the
recommendations given here will modify any requirements of the laboratory standard. This Appendix merely presents
pertinent recommendations from "Prudent Practices", organized into a form convenient for quick reference during
operation of a laboratory facility and during development and application of a Chemical Hygiene Plan. Users of this
appendix should consult "Prudent Practices" for a more extended presentation and justification for each
recommendation.
"Prudent Practices" deal with both safety and chemical hazards while the laboratory standard is concerned primarily
with chemical hazards. Therefore, only those recommendations directed primarily toward control of toxic exposures are
cited in this appendix, with the term "chemical Hygiene" being substituted for the word "safety". However, since
conditions producing or threatening physical injury often pose toxic risks as well, page references concerning major
categories of safety hazards in the laboratory are given in section F.
The recommendations from "Prudent Practices" have been paraphrased, combined, or otherwise reorganized, and
headings have been added. However, their sense has not been changed.
Corresponding Sections of the Standard and this Appendix
The following table is given for the convenience of those who are developing a Chemical Hygiene Plan which will satisfy
the requirements of paragraph (e) of the standard. It indicates those sections of this appendix which are most pertinent
to each of the sections of paragraph (e) and related paragraphs.
___________________________________________________________________
|
| Relevant
Paragraph and topic in laboratory
| appendix
standard
|
section
_____________________________________________________|_____________
|
(e)(3)(i) Standard operating procedures for handling | C, D, E
toxic chemicals.
|
(e)(3)(ii) Criteria to be used for implementation of | D
measures to reduce exposures.
|
(e)(3)(iii) Fume hood performance
| C4b
(e)(3)(iv) Employee information and training
| D10, D9
(including emergency procedures).
|
(e)(3)(v) Requirements for prior approval of
| E2b, E4b
laboratory activities.
|
(e)(3)(vi) Medical consultation and medical
| D5, E4f
examinations.
|
(e)(3)(vii) Chemical hygiene responsibilities.
| B
(e)(3)(viii) Special precautions for work with
| E2, E3, E4
particularly hazardous substances.
|
_____________________________________________________|_____________
In this appendix, those recommendations directed primarily at administrators and supervisors are given in sections A-D.
Those recommendations of primary concern to employees who are actually handling laboratory chemicals are given in
section E. (Reference to page numbers in "Prudent Practices" are given in parentheses.)
76
A. General Principles for Work with Laboratory Chemicals
In addition to the more detailed recommendations listed below in sections B-E, "Prudent Practices" expresses certain
general principles, including the following:
1. It is prudent to minimize all chemical exposures. Because few laboratory chemicals are without hazards, general
precautions for handling all laboratory chemicals should be adopted, rather than specific guidelines for particular
chemicals (2,10). Skin contact with chemicals should be avoided as a cardinal rule (198).
2. Avoid underestimation of risk. Even for substances of no known significant hazard, exposure should be minimized;
for work with substances which present special hazards, special precautions should be taken (10, 37, 38). One should
assume that any mixture will be more toxic than its most toxic component (30, 103) and that all substances of
unknown toxicity are toxic (3, 34).
3. Provide adequate ventilation. The best way to prevent exposure to airborne substances is to prevent their escape
into the working atmosphere by use of hoods and other ventilation devices (32, 198).
4. Institute a chemical hygiene program. A mandatory chemical hygiene program designed to minimize exposures is
needed; it should be a regular, continuing effort, not merely a standby or short-term activity (6,11). Its
recommendations should be followed in academic teaching laboratories as well as by full-time laboratory workers (13).
5. Observe the PELs, TLVs. The Permissible Exposure Limits of OSHA and the Threshold Limit Values of the American
Conference of Governmental Industrial Hygienists should not be exceeded (13).
B. Chemical Hygiene Responsibilities
Responsibility for chemical hygiene rests at all levels (6, 11, 21) including the:
1. Chief executive officer, who has ultimate responsibility for chemical hygiene within the institution and must, with
other administrators, provide continuing support for institutional chemical hygiene (7, 11).
2. Supervisor of the department or other administrative unit, who is responsible for chemical hygiene in that unit (7).
3. chemical hygiene officer(s), whose appointment is essential (7) and who must:
(a) Work with administrators and other employees to develop and implement appropriate chemical hygiene policies and
practices (7);
(b) Monitor procurement, use, and disposal of chemicals used in the lab (8);
(c) See that appropriate audits are maintained (8);
(d) Help project directors develop precautions and adequate facilities (10);
(e) Know the current legal requirements concerning regulated substances (50); and
(f) Seek ways to improve the chemical hygiene program (8, 11).
4. Laboratory supervisor, who has overall responsibility for chemical hygiene in the laboratory (21) including
responsibility to:
(a) Ensure that workers know and follow the chemical hygiene rules, that protective equipment is available and in
77
working order, and that appropriate training has been provided (21, 22);
(b) Provide regular, formal chemical hygiene and housekeeping inspections including routine inspections of emergency
equipment (21, 171);
(c) Know the current legal requirements concerning regulated substances (50, 231);
(d) Determine the required levels of protective apparel and equipment (156, 160, 162); and
(e) Ensure that facilities and training for use of any material being ordered are adequate (215).
5. Project director or director of other specific operation, who has primary responsibility for chemical hygiene
procedures for that operation (7).
6. Laboratory worker, who is responsible for:
(a) Planning and conducting each operation in accordance with the institutional chemical hygiene procedures (7, 21,
22, 230); and
(b) Developing good personal chemical hygiene habits (22).
C. The Laboratory Facility
1. Design. The laboratory facility should have:
(a) An appropriate general ventilation system (see C4 below) with air intakes and exhausts located so as to avoid intake
of contaminated air (194);
(b) Adequate, well-ventilated stockrooms/storerooms (218, 219).
(c) Laboratory hoods and sinks (12, 162);
(d) Other safety equipment including eyewash fountains and drench showers (162, 169); and
(e) Arrangements for waste disposal (12, 240).
2. Maintenance. Chemical-hygiene-related equipment (hoods, incinerator, etc.) should undergo continual appraisal and
be modified if inadequate (11, 12).
3. Usage. The work conducted (10) and its scale (12) must be appropriate to the physical facilities available and,
especially, to the quality of ventilation (13).
4. Ventilation - (a) General laboratory ventilation. This system should: Provide a source of air for breathing and for
input to local ventilation devices (199); it should not be relied on for protection from toxic substances released into the
laboratory (198); ensure that laboratory air is continually replaced, preventing increase of air concentrations of toxic
substances during the working day (194); direct air flow into the laboratory from non-laboratory areas and out to the
exterior of the building (194).
(b) Hoods. A laboratory hood with 2.5 linear feet of hood space per person should be provided for every 2 workers if
they spend most of their time working with chemicals (199); each hood should have a continuous monitoring device to
allow convenient confirmation of adequate hood performance before use (200, 209). If this is not possible, work with
substances of unknown toxicity should be avoided (13) or other types of local ventilation devices should be provided
78
(199). See pp. 201-206 for a discussion of hood design, construction, and evaluation.
(c) Other local ventilation devices. Ventilated storage cabinets, canopy hoods, snorkels, etc. should be provided as
needed (199). Each canopy hood and snorkel should have a separate exhaust duct (207).
(d) Special ventilation areas. Exhaust air from glove boxes and isolation rooms should be passed through scrubbers or
other treatment before release into the regular exhaust system (208). Cold rooms and warm rooms should have
provisions for rapid escape and for escape in the event of electrical failure (209).
(e) Modifications. Any alteration of the ventilation system should be made only if thorough testing indicates that worker
protection from airborne toxic substances will continue to be adequate (12, 193, 204).
(f) Performance. Rate: 4-12 room air changes/hour is normally adequate general ventilation if local exhaust systems
such as hoods are used as the primary method of control (194).
(g) Quality. General air flow should not be turbulent and should be relatively uniform throughout the laboratory, with
no high velocity or static areas (194, 195); airflow into and within the hood should not be excessively turbulent (200);
hood face velocity should be adequate (typically 60-100 lfm) (200, 204).
(h) Evaluation. Quality and quantity of ventilation should be evaluated on installation (202), regularly monitored (at
least every 3 months) (6, 12, 14, 195), and reevaluated whenever a change in local ventilation devices is made (12,
195, 207). See pp 195-198 for methods of evaluation and for calculation of estimated airborne contaminant
concentrations.
D. Components of the Chemical Hygiene Plan
1. Basic Rules and Procedures (Recommendations for these are given in section E, below)
2. Chemical Procurement, Distribution, and Storage
(a) Procurement. Before a substance is received, information on proper handling, storage, and disposal should be
known to those who will be involved (215, 216). No container should be accepted without an adequate identifying label
(216). Preferably, all substances should be received in a central location (216).
(b) Stockrooms/storerooms. Toxic substances should be segregated in a well-identified area with local exhaust
ventilation (221). Chemicals which are highly toxic (227) or other chemicals whose containers have been opened should
be in unbreakable secondary containers (219). Stored chemicals should be examined periodically (at least annually) for
replacement, deterioration, and container integrity (218-19).
Stockrooms/storerooms should not be used as preparation or repackaging areas, should be open during normal
working hours, and should be controlled by one person (219).
(c) Distribution. When chemicals are hand carried, the container should be placed in an outside container or bucket.
Freight-only elevators should be used if possible (223).
(d) Laboratory storage. Amounts permitted should be as small as practical. Storage on bench tops and in hoods is
inadvisable. Exposure to heat or direct sunlight should be avoided. Periodic inventories should be conducted, with
unneeded items being discarded or returned to the storeroom/stockroom (225-6, 229).
3. Environmental Monitoring
Regular instrumental monitoring of airborne concentrations is not usually justified or practical in laboratories but may
79
be appropriate when testing or redesigning hoods or other ventilation devices (12) or when a highly toxic substance is
stored or used regularly (e.g., 3 times/week) (13).
4. Housekeeping, Maintenance, and Inspections
(a) Cleaning. Floors should be cleaned regularly (24).
(b) Inspections. Formal housekeeping and chemical hygiene inspections should be held at least quarterly (6, 21) for
units which have frequent personnel changes and semiannually for others; informal inspections should be continual
(21).
(c) Maintenance. Eye wash fountains should be inspected at intervals of not less than 3 months (6). Respirators for
routine use should be inspected periodically by the laboratory supervisor (169). Other safety equipment should be
inspected regularly. (e.g., every 3-6 months) (6, 24, 171). Procedures to prevent restarting of out-of-service equipment
should be established (25).
(d) Passageways. Stairways and hallways should not be used as storage areas (24). Access to exits, emergency
equipment, and utility controls should never be blocked (24).
5. Medical Program
(a) Compliance with regulations. Regular medical surveillance should be established to the extent required by
regulations (12).
(b) Routine surveillance. Anyone whose work involves regular and frequent handling of toxicologically significant
quantities of a chemical should consult a qualified physician to determine on an individual basis whether a regular
schedule of medical surveillance is desirable (11, 50).
(c) First aid. Personnel trained in first aid should be available during working hours and an emergency room with
medical personnel should be nearby (173). See pp. 176-178 for description of some emergency first aid procedures.
6. Protective Apparel and Equipment
These should include for each laboratory:
(a) Protective apparel compatible with the required degree of protection for substances being handled (158-161);
(b) An easily accessible drench-type safety shower (162, 169);
(c) An eyewash fountain (162)
(d) A fire extinguisher (162-164);
(e) Respiratory protection (164-9), fire alarm and telephone for emergency use (162) should be available nearby; and
(f) Other items designated by the laboratory supervisor (156, 160).
7. Records
(a) Accident records should be written and retained (174).
(b) Chemical Hygiene Plan records should document that the facilities and precautions were compatible with current
80
knowledge and regulations (7).
(c) Inventory and usage records for high-risk substances should be kept as specified in sections E3e below.
(d) Medical records should be retained by the institution in accordance with the requirements of state and federal
regulations (12).
8. Signs and Labels
Prominent signs and labels of the following types should be posted:
(a) Emergency telephone numbers of emergency personnel/facilities, supervisors, and laboratory workers (28);
(b) Identity labels, showing contents of containers (including waste receptacles) and associated hazards (27, 48);
(c) Location signs for safety showers, eyewash stations, other safety and first aid equipment, exits (27) and areas
where food and beverage consumption and storage are permitted (24); and
(d) Warnings at areas or equipment where special or unusual hazards exist (27).
9. Spills and Accidents
(a) A written emergency plan should be established and communicated to all personnel; it should include procedures
for ventilation failure (200), evacuation, medical care, reporting, and drills (172).
(b) There should be an alarm system to alert people in all parts of the facility including isolation areas such as cold
rooms (172).
(c) A spill control policy should be developed and should include consideration of prevention, containment, cleanup, and
reporting (175).
(d) All accidents or near accidents should be carefully analyzed with the results distributed to all who might benefit (8,
28).
10. Information and Training Program
(a) Aim: To assure that all individuals at risk are adequately informed about the work in the laboratory, its risks, and
what to do if an accident occurs (5, 15).
(b) Emergency and Personal Protection Training: Every laboratory worker should know the location and proper use of
available protective apparel and equipment (154, 169).
Some of the full-time personnel of the laboratory should be trained in the proper use of emergency equipment and
procedures (6).
Such training as well as first aid instruction should be available to (154) and encouraged for (176) everyone who might
need it.
(c) Receiving and stockroom/storeroom personnel should know about hazards, handling equipment, protective apparel,
and relevant regulations (217).
(d) Frequency of Training: The training and education program should be a regular, continuing activity - not simply an
81
annual presentation (15).
(e) Literature/Consultation: Literature and consulting advice concerning chemical hygiene should be readily available to
laboratory personnel, who should be encouraged to use these information resources (14).
11. Waste Disposal Program.
(a) Aim: To assure that minimal harm to people, other organisms, and the environment will result from the disposal of
waste laboratory chemicals (5).
(b) Content (14, 232, 233, 240): The waste disposal program should specify how waste is to be collected, segregated,
stored, and transported and include consideration of what materials can be incinerated. Transport from the institution
must be in accordance with DOT regulations (244).
(c) Discarding Chemical Stocks: Unlabeled containers of chemicals and solutions should undergo prompt disposal; if
partially used, they should not be opened (24, 27).
Before a worker's employment in the laboratory ends, chemicals for which that person was responsible should be
discarded or returned to storage (226).
(d) Frequency of Disposal: Waste should be removed from laboratories to a central waste storage area at least once
per week and from the central waste storage area at regular intervals (14).
(e) Method of Disposal: Incineration in an environmentally acceptable manner is the most practical disposal method for
combustible laboratory waste (14, 238, 241).
Indiscriminate disposal by pouring waste chemicals down the drain (14, 231, 242) or adding them to mixed refuse for
landfill burial is unacceptable (14).
Hoods should not be used as a means of disposal for volatile chemicals (40, 200).
Disposal by recycling (233, 243) or chemical decontamination (40, 230) should be used when possible.
E. Basic Rules and Procedures for Working with Chemicals
The Chemical Hygiene Plan should require that laboratory workers know and follow its rules and procedures. In
addition to the procedures of the sub programs mentioned above, these should include the rules listed below.
1. General Rules
The following should be used for essentially all laboratory work with chemicals:
(a) Accidents and spills - Eye Contact: Promptly flush eyes with water for a prolonged period (15 minutes) and seek
medical attention (33, 172).
Ingestion: This is one route of entry for which treatment depends on the type and amount of chemical involved. Seek
medical attention immediately.
Skin Contact: Promptly flush the affected area with water (33, 172, 178) and remove any contaminated clothing (172,
178). If symptoms persist after washing, seek medical attention (33).
Clean-up. Promptly clean up spills, using appropriate protective apparel and equipment and proper disposal (24, 33).
82
See pp. 233-237 for specific clean-up recommendations.
(b) Avoidance of "routine" exposure: Develop and encourage safe habits (23); avoid unnecessary exposure to
chemicals by any route (23);
Do not smell or taste chemicals (32). Vent apparatus which may discharge toxic chemicals (vacuum pumps, distillation
columns, etc.) into local exhaust devices (199).
Inspect gloves (157) and test glove boxes (208) before use.
Do not allow release of toxic substances in cold rooms and warm rooms, since these have contained recirculated
atmospheres (209).
(c) Choice of chemicals: Use only those chemicals for which the quality of the available ventilation system is appropriate
(13).
(d) Eating, smoking, etc.: Avoid eating, drinking, smoking, gum chewing, or application of cosmetics in areas where
laboratory chemicals are present (22, 24, 32, 40); wash hands before conducting these activities (23, 24).
Avoid storage, handling, or consumption of food or beverages in storage areas, refrigerators, glassware or utensils
which are also used for laboratory operations (23, 24, 226).
(e) Equipment and glassware: Handle and store laboratory glassware with care to avoid damage; do not use damaged
glassware (25). Use extra care with Dewar flasks and other evacuated glass apparatus; shield or wrap them to contain
chemicals and fragments should implosion occur (25). Use equipment only for its designed purpose (23, 26).
(f) Exiting: Wash areas of exposed skin well before leaving the laboratory (23).
(g) Horseplay: Avoid practical jokes or other behavior which might confuse, startle or distract another worker (23).
(h) Mouth suction: Do not use mouth suction for pipeting or starting a siphon (23, 32).
(i) Personal apparel: Confine long hair and loose clothing (23, 158). Wear shoes at all times in the laboratory but do not
wear sandals, perforated shoes, or sneakers (158).
(j) Personal housekeeping: Keep the work area clean and uncluttered, with chemicals and equipment being properly
labeled and stored; clean up the work area on completion of an operation or at the end of each day (24).
(k) Personal protection: Assure that appropriate eye protection (154-156) is worn by all persons, including visitors,
where chemicals are stored or handled (22, 23, 33, 154).
Wear appropriate gloves when the potential for contact with toxic materials exists (157); inspect the gloves before each
use, wash them before removal, and replace them periodically (157). (A table of resistance to chemicals of common
glove materials is given p. 159).
Use appropriate (164-168) respiratory equipment when air contaminant concentrations are not sufficiently restricted by
engineering controls (164-5), inspecting the respirator before use (169).
Use any other protective and emergency apparel and equipment as appropriate (22, 157-162).
Avoid use of contact lenses in the laboratory unless necessary; if they are used, inform supervisor so special
83
precautions can be taken (155).
Remove laboratory coats immediately on significant contamination (161).
(l) Planning: Seek information and advice about hazards (7), plan appropriate protective procedures, and plan
positioning of equipment before beginning any new operation (22, 23).
(m) Unattended operations: Leave lights on, place an appropriate sign on the door, and provide for containment of
toxic substances in the event of failure of a utility service (such as cooling water) to an unattended operation (27, 128).
(n) Use of hood: Use the hood for operations which might result in release of toxic chemical vapors or dust (198-9).
As a rule of thumb, use a hood or other local ventilation device when working with any appreciably volatile substance
with a TLV of less than 50 ppm (13).
Confirm adequate hood performance before use; keep hood closed at all times except when adjustments within the
hood are being made (200); keep materials stored in hoods to a minimum and do not allow them to block vents or air
flow (200).
Leave the hood "on" when it is not in active use if toxic substances are stored in it or if it is uncertain whether adequate
general laboratory ventilation will be maintained when it is "off" (200).
(o) Vigilance: Be alert to unsafe conditions and see that they are corrected when detected (22).
(p) Waste disposal: Assure that the plan for each laboratory operation includes plans and training for waste disposal
(230).
Deposit chemical waste in appropriately labeled receptacles and follow all other waste disposal procedures of the
Chemical Hygiene Plan (22, 24).
Do not discharge to the sewer concentrated acids or bases (231); highly toxic, malodorous, or lachrymatory substances
(231); or any substances which might interfere with the biological activity of waste water treatment plants, create fire
or explosion hazards, cause structural damage or obstruct flow (242).
(q) Working alone: Avoid working alone in a building; do not work alone in a laboratory if the procedures being
conducted are hazardous (28).
2. Working with Allergens and Embryotoxins
(a) Allergens (examples: diazomethane, isocyanates, bichromates): Wear suitable gloves to prevent hand contact with
allergens or substances of unknown allergenic activity (35).
(b) Embryotoxins (34-5) (examples: organomercurials, lead compounds, formamide): If you are a woman of
childbearing age, handle these substances only in a hood whose satisfactory performance has been confirmed, using
appropriate protective apparel (especially gloves) to prevent skin contact.
Review each use of these materials with the research supervisor and review continuing uses annually or whenever a
procedural change is made.
Store these substances, properly labeled, in an adequately ventilated area in an unbreakable secondary container.
84
Notify supervisors of all incidents of exposure or spills; consult a qualified physician when appropriate.
3. Work with Chemicals of Moderate Chronic or High Acute Toxicity
Examples: diisopropylfluorophosphate (41), hydrofluoric acid (43), hydrogen cyanide (45).
Supplemental rules to be followed in addition to those mentioned above (Procedure B of "Prudent Practices", pp. 3941):
(a) Aim: To minimize exposure to these toxic substances by any route using all reasonable precautions (39).
(b) Applicability: These precautions are appropriate for substances with moderate chronic or high acute toxicity used in
significant quantities (39).
(c) Location: Use and store these substances only in areas of restricted access with special warning signs (40, 229).
Always use a hood (previously evaluated to confirm adequate performance with a face velocity of at least 60 linear feet
per minute) (40) or other containment device for procedures which may result in the generation of aerosols or vapors
containing the substance (39); trap released vapors to revent their discharge with the hood exhaust (40).
(d) Personal protection: Always avoid skin contact by use of gloves and long sleeves (and other protective apparel as
appropriate) (39). Always wash hands and arms immediately after working with these materials (40).
(e) Records: Maintain records of the amounts of these materials on hand, amounts used, and the names of the workers
involved (40, 229).
(f) Prevention of spills and accidents: Be prepared for accidents and spills (41).
Assure that at least 2 people are present at all times if a compound in use is highly toxic or of unknown toxicity (39).
Store breakable containers of these substances in chemically resistant trays; also work and mount apparatus above
such trays or cover work and storage surfaces with removable, absorbent, plastic backed paper (40).
If a major spill occurs outside the hood, evacuate the area; assure that cleanup personnel wear suitable protective
apparel and equipment (41).
(g) Waste: Thoroughly decontaminate or incinerate contaminated clothing or shoes (41). If possible, chemically
decontaminate by chemical conversion (40).
Store contaminated waste in closed, suitably labeled, impervious containers (for liquids, in glass or plastic bottles halffilled with vermiculite) (40).
4. Work with Chemicals of High Chronic Toxicity
(Examples: dimethylmercury and nickel carbonyl (48), benzo-a-pyrene (51), N-nitrosodiethylamine (54), other human
carcinogens or substances with high carcinogenic potency in animals (38).)
Further supplemental rules to be followed, in addition to all these mentioned above, for work with substances of known
high chronic toxicity (in quantities above a few milligrams to a few grams, depending on the substance) (47).
(Procedure A of "Prudent Practices" pp. 47-50).
(a) Access: Conduct all transfers and work with these substances in a "controlled area": a restricted access hood, glove
85
box, or portion of a lab, designated for use of highly toxic substances, for which all people with access are aware of the
substances being used and necessary precautions (48).
(b) Approvals: Prepare a plan for use and disposal of these materials and obtain the approval of the laboratory
supervisor (48).
(c) Non-contamination/Decontamination: Protect vacuum pumps against contamination by scrubbers or HEPA filters
and vent them into the hood (49). Decontaminate vacuum pumps or other contaminated equipment, including
glassware, in the hood before removing them from the controlled area (49, 50).
Decontaminate the controlled area before normal work is resumed there (50).
(d) Exiting: On leaving a controlled area, remove any protective apparel (placing it in an appropriate, labeled container)
and thoroughly wash hands, forearms, face, and neck (49).
(e) Housekeeping: Use a wet mop or a vacuum cleaner equipped with a HEPA filter instead of dry sweeping if the toxic
substance was a dry powder (50).
(f) Medical surveillance: If using toxicologically significant quantities of such a substance on a regular basis (e.g., 3
times per week), consult a qualified physician concerning desirability of regular medical surveillance (50).
(g) Records: Keep accurate records of the amounts of these substances stored (229) and used, the dates of use, and
names of users (48).
(h) Signs and labels: Assure that the controlled area is conspicuously marked with warning and restricted access signs
(49) and that all containers of these substances are appropriately labeled with identity and warning labels (48).
(i) Spills: Assure that contingency plans, equipment, and materials to minimize exposures of people and property in
case of accident are available (233-4).
(j) Storage: Store containers of these chemicals only in a ventilated, limited access (48, 227, 229) area in appropriately
labeled, unbreakable, chemically resistant, secondary containers (48, 229).
(k) Glove boxes: For a negative pressure glove box, ventilation rate must be at least 2 volume changes/hour and
pressure at least 0.5 inches of water (48). For a positive pressure glove box, thoroughly check for leaks before each
use (49). In either case, trap the exit gases or filter them through a HEPA filter and then release them into the hood
(49).
(l) Waste: Use chemical decontamination whenever possible; ensure that containers of contaminated waste (including
washings from contaminated flasks) are transferred from the controlled area in a secondary container under the
supervision of authorized personnel (49, 50, 233).
5. Animal Work with Chemicals of High Chronic Toxicity
(a) Access: For large scale studies, special facilities with restricted access are preferable (56).
(b) Administration of the toxic substance: When possible, administer the substance by injection or gavage instead of in
the diet. If administration is in the diet, use a caging system under negative pressure or under laminar air flow directed
toward HEPA filters (56).
(c) Aerosol suppression: Devise procedures which minimize formation and dispersal of contaminated aerosols, including
those from food, urine, and feces (e.g., use HEPA filtered vacuum equipment for cleaning, moisten contaminated
86
bedding before removal from the cage, mix diets in closed containers in a hood) (55, 56).
(d) Personal protection: When working in the animal room, wear plastic or rubber gloves, fully buttoned laboratory coat
or jumpsuit and, if needed because of incomplete suppression of aerosols, other apparel and equipment (shoe and
head coverings, respirator) (56).
(e) Waste disposal: Dispose of contaminated animal tissues and excreta by incineration if the available incinerator can
convert the contaminant to non-toxic products (238); otherwise, package the waste appropriately for burial in an EPAapproved site (239).
F. Safety Recommendations
The above recommendations from "Prudent Practices" do not include those which are directed primarily toward
prevention of physical injury rather than toxic exposure. However, failure of precautions against injury will often have
the secondary effect of causing toxic exposures. Therefore, we list below page references for recommendations
concerning some of the major categories of safety hazards which also have implications for chemical hygiene:
1.
2.
3.
4.
5.
Corrosive agents: (35-6)
Electrically powered laboratory apparatus: (179-92)
Fires, explosions: (26, 57-74, 162-64, 174-5, 219-20, 226-7)
Low temperature procedures: (26, 88)
Pressurized and vacuum operations (including use of compressed gas cylinders): (27, 75-101)
G. Material Safety Data Sheets
Material safety data sheets are presented in "Prudent Practices" for the chemicals listed below. (Asterisks denote that
comprehensive material safety data sheets are provided).
* Acetyl peroxide (105)
* Acrolein (106)
* Acrylonitrile
Ammonia (anhydrous)(91)
* Aniline (109)
* Benzene (110)
* Benzo[a]pyrene (112)
* Bis(chloromethyl) ether (113)
Boron trichloride (91)
Boron trifluoride (92)
Bromine (114)
* Tert-butyl hydroperoxide (148)
* Carbon disulfide (116)
Carbon monoxide (92)
* Carbon tetrachloride (118)
* Chlorine (119)
Chlorine trifluoride (94)
* Chloroform (121)
Chloromethane (93)
* Diethyl ether (122)
Diisopropyl fluorophosphate (41)
* Dimethylformamide (123)
* Dimethyl sulfate (125)
* Dioxane (126)
* Ethylene dibromide (128)
* Fluorine (95)
87
* Formaldehyde (130)
* Hydrazine and salts (132)
Hydrofluoric acid (43)
Hydrogen bromide (98)
Hydrogen chloride (98)
* Hydrogen cyanide (133)
* Hydrogen sulfide (135)
Mercury and compounds (52)
* Methanol (137)
* Morpholine (138)
* Nickel carbonyl (99)
* Nitrobenzene (139)
Nitrogen dioxide (100)
N-nitrosodiethylamine (54)
* Peracetic acid (141)
* Phenol (142)
* Phosgene (143)
* Pyridine (144)
* Sodium azide (145)
* Sodium cyanide (147)
Sulfur dioxide (101)
* Trichloroethylene (149)
* Vinyl chloride (150)
[76 FR 33609, June 8, 2011]
• Part Number:
1910
• Part Title:
Occupational Safety and Health Standards
• Subpart:
Z
• Subpart Title:
Toxic and Hazardous Substances
• Standard Number:
1910.1450 App B
• Title:
References (Non-Mandatory)
The following references are provided to assist the employer in the development of a Chemical Hygiene Plan. The
materials listed below are offered as non-mandatory guidance. References listed here do not imply specific
endorsement of a book, opinion, technique, policy or a specific solution for a safety or health problem. Other references
not listed here may better meet the needs of a specific laboratory. (a) Materials for the development of the Chemical
Hygiene Plan:
1. American Chemical Society, Safety in Academic Chemistry Laboratories, 4th edition, 1985.
2. Fawcett, H.H. and W.S. Wood, Safety and Accident Prevention in Chemical Operations, 2nd edition, WileyInterscience, New York, 1982.
3. Flury, Patricia A., Environmental Health and Safety in the Hospital Laboratory, Charles C. Thomas Publisher,
Springfield IL, 1978.
4. Green, Michael E. and Turk, Amos, Safety in Working with Chemicals, Macmillan Publishing Co., NY, 1978.
88
5. Kaufman, James A., Laboratory Safety Guidelines, Dow Chemical Co., Box 1713, Midland, MI 48640, 1977.
6. National Institutes of Health, NIH Guidelines for the Laboratory use of Chemical Carcinogens, NIH Pub. No. 81-2385,
GPO, Washington, DC 20402, 1981.
7. National Research Council, Prudent Practices for Disposal of Chemicals from Laboratories, National Academy Press,
Washington, DC, 1983.
8. National Research Council, Prudent Practices for Handling Hazardous Chemicals in Laboratories, National Academy
Press, Washington, DC, 1981.
9. Renfrew, Malcolm, Ed., Safety in the Chemical Laboratory, Vol. IV, J. Chem. Ed., American Chemical Society, Easlon,
PA, 1981.
10. Steere, Norman V., Ed., Safety in the Chemical Laboratory, J. Chem. Ed. American Chemical Society, Easlon, PA,
18042, Vol. I, 1967, Vol. II, 1971, Vol. III, 1974.
11. Steere, Norman V., Handbook of Laboratory Safety, the Chemical Rubber Company Cleveland, OH, 1971.
12. Young, Jay A., Ed., Improving Safety in the Chemical Laboratory, John Wiley & Sons, Inc. New York, 1987.
(b) Hazardous Substances Information:
1. American Conference of Governmental Industrial Hygienists, Threshold Limit Values for Chemical Substances and
Physical Agents in the Workroom Environment with Intended Changes, 6500 Glenway Avenue, Bldg. D-7, Cincinnati, OH
45211-4438.
2. Annual Report on Carcinogens, National Toxicology Program U.S. Department of Health and Human Services, Public
Health Service, U.S. Government Printing Office, Washington, DC, (latest edition).
3. Best Company, Best Safety Directory, Vols. I and II, Oldwick, N.J., 1981.
4. Bretherick, L., Handbook of Reactive Chemical Hazards, 2nd edition, Butterworths, London, 1979.
5. Bretherick, L., Hazards in the Chemical Laboratory, 3rd edition, Royal Society of Chemistry, London, 1986.
6. Code of Federal Regulations, 29 CFR part 1910 subpart Z. U.S. Govt. Printing Office, Washington, DC 20402 (latest
edition).
7. IARC Monographs on the Evaluation of the Carcinogenic Risk of chemicals to Man, World Health Organization
Publications Center, 49 Sheridan Avenue, Albany, New York 12210 (latest editions).
8. NIOSH/OSHA Pocket Guide to Chemical Hazards. NIOSH Pub. No. 85-114, U.S. Government Printing Office,
Washington, DC, 1985 (or latest edition).
9. Occupational Health Guidelines, NIOSH/OSHA. NIOSH Pub. No. 81-123 U.S. Government Printing Office, Washington,
DC, 1981.
10. Patty, F.A., Industrial Hygiene and Toxicology, John Wiley & Sons, Inc., New York, NY (Five Volumes).
11. Registry of Toxic Effects of Chemical Substances, U.S. Department of Health and Human Services, Public Health
Service, Centers for Disease Control, National Institute for Occupational Safety and Health, Revised Annually, for sale
89
from Superintendent of documents US. Govt. Printing Office, Washington, DC 20402.
12. The Merck Index: An Encyclopedia of Chemicals and Drugs. Merck and Company Inc. Rahway, N.J., 1976 (or latest
edition).
13. Sax, N.I. Dangerous Properties of Industrial Materials, 5th edition, Van Nostrand Reinhold, NY., 1979.
14. Sittig, Marshall, Handbook of Toxic and Hazardous Chemicals, Noyes Publications. Park Ridge, NJ, 1981.
(c) Information on Ventilation:
1. American Conference of Governmental Industrial Hygienists Industrial Ventilation (latest edition), 6500 Glenway
Avenue, Bldg. D-7, Cincinnati, Ohio 45211-4438.
2. American National Standards Institute, Inc. American National Standards Fundamentals Governing the Design and
Operation of Local Exhaust Systems ANSI Z 9.2-1979 American National Standards Institute, N.Y. 1979.
3. Imad, A.P. and Watson, C.L. Ventilation Index: An Easy Way to Decide about Hazardous Liquids, Professional Safety
pp 15-18, April 1980.
4. National Fire Protection Association, Fire Protection for Laboratories Using Chemicals NFPA-45, 1982.
Safety Standard for Laboratories in Health Related Institutions, NFPA, 56c, 1980.
Fire Protection Guide on Hazardous Materials, 7th edition, 1978.
National Fire Protection Association, Batterymarch Park, Quincy, MA 02269.
5. Scientific Apparatus Makers Association (SAMA), Standard for Laboratory Fume Hoods, SAMA LF7-1980, 1101 16th
Street, NW., Washington, DC 20036.
(d) Information on Availability of Referenced Material:
1. American National Standards Institute (ANSI), 1430 Broadway, New York, NY 10018.
2. American Society for Testing and Materials (ASTM), 1916 Race Street, Philadelphia, PA 19103.
[55 FR 3327, Jan. 31, 1990; 57 FR 29204, July 1, 1992; 61 FR 5507, Feb. 13, 1996]
90