Energy (KJ/mol)
advertisement
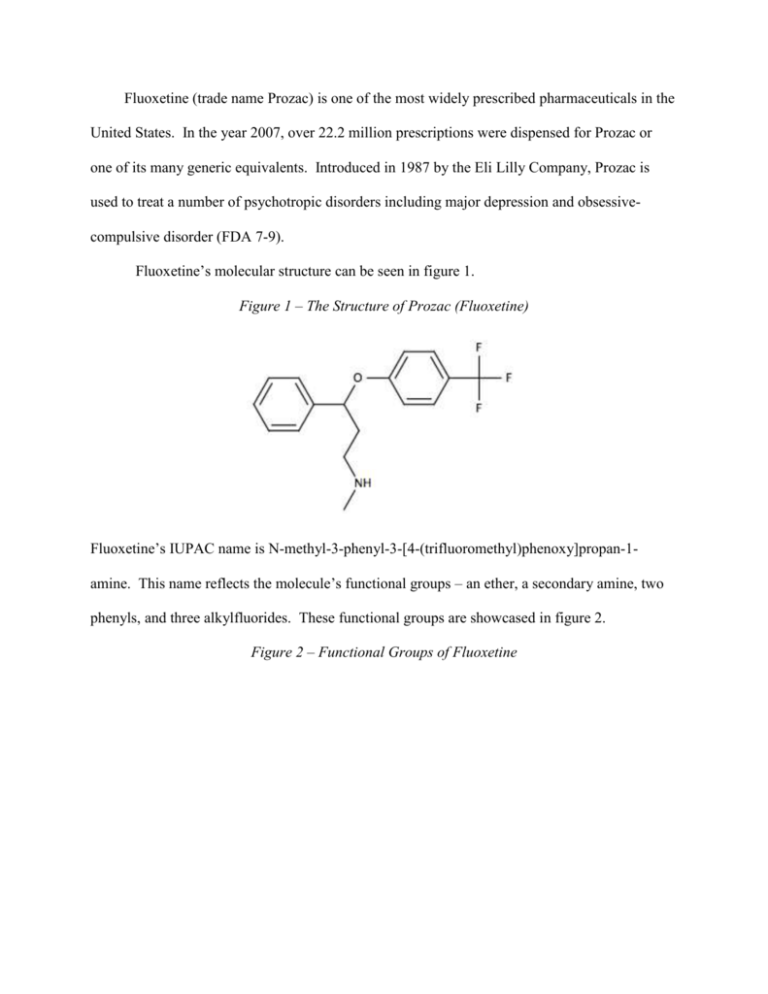
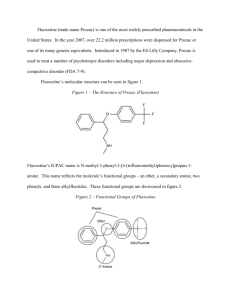
Fluoxetine (trade name Prozac) is one of the most widely prescribed pharmaceuticals in the United States. In the year 2007, over 22.2 million prescriptions were dispensed for Prozac or one of its many generic equivalents. Introduced in 1987 by the Eli Lilly Company, Prozac is used to treat a number of psychotropic disorders including major depression and obsessivecompulsive disorder (FDA 7-9). Fluoxetine’s molecular structure can be seen in figure 1. Figure 1 – The Structure of Prozac (Fluoxetine) Fluoxetine’s IUPAC name is N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]propan-1amine. This name reflects the molecule’s functional groups – an ether, a secondary amine, two phenyls, and three alkylfluorides. These functional groups are showcased in figure 2. Figure 2 – Functional Groups of Fluoxetine 2 In addition to possessing several varied functional groups, fluoxetine also contains seven different types of covalent bonds. These include highly stable bonds such as the conjugated carbon-to-carbon double bonds of the phenyl groups and relatively unstable bonds such as the carbon-to-nitrogen single bonds of the amine. All seven bond types, bond lengths, and associated energies are given in figure 3. Figure 3 – Bond Types in Fluoxetine C=C C−C C−F C−H C−N C−O H−N Count 6 10 3 17 2 2 1 Length (Å) 1.4 1.4 1.4 1.1 1.4 1.4 1.0 Energy (KJ/mol) 602 346 485 411 305 358 386 Fluoxetine has received a Chemical Abstracts Service registry number of 54910-89-3. Its molecular formula is C17H18F3NO, and its molar mass is 309.33 g/mol (O’Neil 4185). Under standard laboratory conditions, Fluoxetine is a white to off-white crystalline solid. Pure samples exhibit a melting point of 158.4-158.9 degrees Celsius. With a maximum aqueous solubility of 3 14 mg/mL, fluoxetine is practically insoluble in water. Fluoxetine’s LD50 for mice is 248 mg/kg, and for rats it is 452 mg/kg (O’Neil 4185). Fluoxetine’s third carbon is the compound’s only stereogenic center. For this reason, the two structures seen on the following page in figure 4 are the compound’s only two enantiomers. Figure 4 – The Enantiomers of Fluoxetine Therapeutic preparations of fluoxetine are racemic mixtures of (R)-fluoxetine and (S)-fluoxetine. Both enantiomers are highly specific and potent serotonin reuptake inhibitors; however, (S)fluoxetine is eliminated from the body more slowly than its (R) counterpart. For this reason, the predominant enantiomer found in blood plasma is (S)-fluoxetine (FDA 2). Fluoxetine’s 1HNMR spectrum is given in figure 5 below. Figure 5 – 1HNMR Spectrum of Fluoxetine 4 (a) (a) (d) (b) (c) (e) (d) (c) (a) (b) (e) Several multiplets (a) are present in the 7.0-7.8 ppm region. These signals correspond to the protons of the two aromatic rings. A triplet (b) can be seen at 5.20 ppm. This downfield signal corresponds to the highly deshielded proton of the third carbon on fluoxetine’s aliphatic carbon chain. A second triplet (c) is located at 2.68 ppm. This signal corresponds to the two protons of the first carbon of the compound’s aliphatic carbon chain. The singlet (d) appearing at 2.43 ppm corresponds to the three protons of the amine’s methyl group. Finally, the doublet of triplets (e) at 2.07 ppm corresponds to the protons of the second carbon of fluoxetine’s aliphatic carbon chain. A portion of Fluoxetine’s infrared spectrum can be seen in figure 6 below. Figure 6 – Infrared Spectrum of Fluoxetine 5 Fluoxetine’s IR spectrum likely contains a medium peak at 3500 cm-1 corresponding to N–H stretching of the secondary amine. Aromatic C−H stretching likely occurs as a strong, sharp peak at 3150-3050 cm-1. A strong, sharp peak corresponding to aliphatic Csp3−H stretching should be visible at 3000-2850 cm-1. As can actually be seen in figure 6, aromatic C=C stretching appears as a series of sharp, medium-to-strong peaks between 1600 and 1400 cm-1. Additionally, stretching of the C–O bonds of the ether produce a strong, sharp signal at 1300 cm-1. Finally, the three C–F bonds can be seen as strong signals between 1400 cm-1 and 1000 cm-1 (Pavia, Lampman, Kriz, and Engel 851). While several viable syntheses exist for fluoxetine, one particular synthesis is especially suited for undergraduate laboratories. While some syntheses use toxic compounds such as the reducing agent diborane (B2H6) and the chlorinating agent thionyl chloride (SOCl2) to yield fluoxetine, there is at least one synthesis that circumvents these hazardous reagents. The synthesis given by Perrine, Sabanayagam, and Reynolds instead uses the less hazardous reagents sodium borohydride (NaBH4) and potassium tert-butoxide (KOC[CH3]3). Furthermore, while 6 other syntheses require use of the rather costly compound 4-(trifluoromethyl)phenol, this synthesis avoids the compound and instead utilizes the inexpensive chemical 1-chloro-4(trifluoromethyl)benzene. For these reasons, the synthesis given in figure 7 is best suited for introductory organic chemistry laboratories. Figure 7 – A Synthesis of Fluoxetine 1 2 3 4 5 The commercially available compound 3-(dimethylamino)-1-phenylpropan-1-one (1) is reduced with sodium borohydride (NaBH4) to form 3-(dimethylamino)-1-phenylpropan-1-ol (2). This secondary alcohol is deprotonated with potassium tert-butoxide (KOC[CH3]3) and undergoes aromatic substitution onto 1-chloro-4-(trifluoromethyl)benzene (3) via the loss of a chloride ion. This aromatic substitution occurs within the polar aprotic solvent DMAA and subsequently yields the drug precursor “N-methyl-Prozac” (4). NMP is converted to Prozac (fluoxetine, 5) via N-demethylation with cyanobromide (CNBr). Fluoxetine’s main activity and mechanism of action in vivo is as a selective serotonin reuptake inhibitor (SSRI). Serotonin, 5-hydroxytryptamine (5-HT), is a neurotransmitter associated with the mental sensation of well-being. In healthy neurons, 5-hydroxytryptamine is synthesized from the amino acid tryptophan via catalysis by tryptophanhydroxylase (TH) and aromatic aminoacid decarboxylase (AADC). Newly generated serotonin is stored in vesicles in the axon terminal and then released into the synaptic space. The freed serotonin molecules 7 activate 5-HT receptors on the adjacent dendrites. Over time, the serotonin in the synaptic space is either degraded by monoamine oxidase or collected by the 5-HT transporters on the axon. In the latter case, the serotonin is placed in vesicles and reused. A significant contributing factor to major depression is the overactive reuptake of serotonin. If the 5-HT transporters remove serotonin too quickly, neurological imbalances including depression can occur. Fluoxetine and other selective serotonin reuptake inhibitors block a portion of the 5-HT transporters and increase the serotonin available to the dendritic 5-HT receptors. This mechanism of selectively inhibiting serotonin reuptake can restore neurological balance and reduce the symptoms of depression (Wong, Perry, and Bymaster 767). In addition to the above model, a new non-SSRI mechanism of action for fluoxetine has recently been discovered by medicinal chemists. In 2006, Pinna and co-workers reported that fluoxetine and its N-desmethyl congener norfluoxetine selectively increase brain neurosteroid content at doses inadequate to inhibit serotonin reuptake. Pinna believes that fluoxetine’s pharmacologic actions would be described more accurately as a “selective brain steroidogenic stimulant.” This pharmacology is consistent with neurosteroids’ known involvement in human brain pathophysiology, such as major depression, postpartum depression, post-traumatic stress disorder, and various anxiety disorders (Daniels 1039). Consequently, Prozac’s health effects seem to be derived from not only its ability to selectively inhibit serotonin reuptake, but also its ability to increase brain neurosteroids. Fluoxetine is indicated for a number of psychotropic disorders. The drug’s most common indication is for the treatment of major depressive disorder in adults. This disorder is characterized by a prominent and persistent depressed or dysphoric mood that significantly affects the ability of the individual to function. Depression in children and adolescents has also been effectively treated with Prozac (FDA 7-8). 8 Another indication for this prominent antidepressant is in the treatment of obsessivecompulsive disorder. Individuals with this disorder suffer from obsessions or compulsions that cause marked distress, consume vast amounts of time, or interfere significantly with day-to-day activities. Prozac’s effectiveness in the treatment of OCD has been firmly established via 13week trials with adults as well as with pediatric patients. Fluoxetine is also indicated to treat bulimia nervosa. Bulimia, a behavioral disorder characterized by binge eating and subsequent purging, has been treated successfully with Prozac. Other uses for this SSRI include the treatment of panic disorders and premenstrual dysphoric disorder (FDA 8-9). Because of the relatively low incidence of adverse effects, fluoxetine has gained widespread use in the past twenty years. However, a few adverse effects should be noted. These effects include nausea (24% incidence), nervousness, insomnia (15% incidence), sedation, headache, dizziness, dry mouth, diarrhea, constipation, sweating, tremor, anorexia, and sexual dysfunction. Occurring in 3% if patients, rashes signal a potentially serious side effect and warrant immediate discontinuation of the medicine. Overall, roughly 15% of all patients prescribed fluoxetine will discontinue its use due to adverse side effects (Stokes 1191). Fluoxetine is contraindicated in a few important situations. This pharmaceutical is not to be prescribed to any patient with a known allergy to SSRI’s. Since SSRIs can cause lifethreatening allergic reactions, treatment should be immediately discontinued if the patient develops a rash. Potentially fatal reactions have also occurred with concomitant use of monoamine oxidase inhibitors, thioridazine, or pimozide with fluoxetine. Therefore, patients should discontinue usage of these medications at least two weeks before taking the first dose of fluoxetine. Additionally, fluoxetine should be discontinued at least five weeks before starting treatment with either monoamine oxidase inhibitors, thioridazine, or pimozide with fluoxetine (NIH 3). Herbal medications such as St. John’s wort or tryptophan should be avoided while 9 taking fluoxetine. Since both of these over-the-counter medications raise serotonin levels, a serious condition known as serotonin syndrome may occur (Mayo 3). In addition, taking fluoxetine by expecting mothers has been shown to prove damaging to unborn infants. A case study published in 2006, showed that infants whose mothers took SSRIs after the twentieth week of pregnancy were more likely to have persistent pulmonary hypertension (PPHN), a cause of morbidity in infants (FDA). Abrupt discontinuation of this drug may cause adverse CNS effects, and should be tapered off. This pharmaceutical may cause weight loss and should not be administered to patients with anorexia nervosa, or in the case where weight loss is not desirable. Fluoxetine is not recommended for those patients with cirrhosis because the elimination half-life is prolonged, making the compound persist in the blood stream. Pediatric fluoxetine prescriptions should only be reserved for children, having more than seven years, with obsessive compulsive disorder; fluoxetine should not be used in pediatrics for any other psychiatric disorder (RX-s). When fluoxetine was introduced in 1987, it enabled a monumental step forward in the treatment of depression. Fluoxetine treats depression more effectively than its predecessors with significantly fewer and more benign side effects. Due to the ease of once-a-day dosing and a relatively low side effect profile, patient compliance is high with fluoxetine (Stokes 1224). A truly revolutionary treatment for mood disorders, fluoxetine is a pharmaceutical drug the authors would readily prescribe for patients suffering from major depression, obsessive-compulsive disorder, or other psychological conditions related to serotonin imbalance. Works Cited Daniels, R. Nathan and Craig Lindsley. “A New, Non-SSRI Mechanism of Action for Prozac.” Current Topics in Medicinal Chemistry 7 (2007): 1039. “Fluoxetine Contraindictions and Precautions.”RX-s.net. Apr. 2005. 25 Mar. 2011 <http://rxs.net/weblog/more/fluoxetine_contraindications_and_precautions/>. 10 Food and Drug Administration. Prozac. Feb. 2004. 22 Mar. 2011<http://www.fda.gov/ohrms/ dockets/ac/04/briefing/4006B1_05_Prozac-label.pdf>. “Information for Healthcare Professionals: Fluoxetine (marketed as Prozac)”. Jul. 2006. 07 Apr. 2011< http://alturl.com/vbvkp>. Lide, David R. CRC Handbook of Chemistry and Physics: a Ready-reference Book of Chemical and Physical Data: 2008-2009. 90th ed. Boca Raton: CRC, 2009. Mayo Clinic Staff. “Selective Serotonin Reuptake Inhibitors.” Mayo Clinic News. Dec. 2010. 25 March 2011 <http://vsearch.nlm.nih.gov/vivisimo/cgi-bin/query-meta?v%3Aproject= medlineplus&query=prozac&x=12&y=16.html>. National Institute of Health. Fluoxetine. March 2011. 22 Mar. 2011<http://www.nlm.nih.gov/ medlineplus/druginfo/meds/a689006.html>. O’Neil, Maryadele, Patricia Heckelman, Cherie Koch, and Kristin Roman, eds. The Merck Index. 14th ed. Whitehouse Station: Merck Research Laboratories, 2006. Pavia, Donald L., Gary M. Lampman, George S. Kriz, and Randall G. Engel. Introduction to Organic Laboratory Techniques: a Microscale Approach. Belmont: Thomson Brooks/Cole, 2007. Perrine, Daniel M., Nathan R. Sabanayagam, and Kristy J. Reynolds. “The Synthesis of NMP, a Fluoxetine (Prozac) Precursor, in the Introductory Organic Laboratory.” In the Laboratory: Notes for the Instructor. Stokes, Peter E. and Aliza Holtz. “Fluoxetine Tenth Anniversary Update: The Progress Continues.” Clinical Therapeutics 19 (1997): 1137-1250. Wong, David T., Kenneth W. Perry, and Frank Porter Bymaster. "Access: The Discovery of Fluoxetine Hydrochloride (Prozac): Nature Reviews Drug Discovery." Nature Publishing 11 Group: Science Journals, Jobs, and Information. Web. 10 Mar. 2011. <http://www.nature.com/nrd/journal/v4/n9/authors/nrd1821.html>.