chemistry - NAF Directorate of Education
advertisement

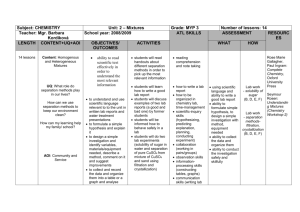
CHEMISTRY FIRST TERM SS 1 WEEK (a) 1 TOPIC / CONTENT (b) INTRODUCTION TO CHEMISTRY 1. Meaning of chemistry 2. Career prospects tied to chemistry 3. Applications (Hospital, Military, Teaching, Chemical and Petrochemical Industries, Space Science, Agriculture etc). 2 INTRODUCTION TO CHEMISTRY 1. Adverse effects of chemicals, drug abuse, poisoning, corrosion, pollution. 2. Scientific method. 3 CHEMICAL INDUSTRIES 1. Types of chemical industries 2. Importance: - to the individual - to the nation. ACTIVITIES (c) Teacher: - Defines chemistry, gives examples and illustrations. - Guide students to identify career prospect in chemistry. - Uses posters and charts to guide class discussion. Instructional Resources: - Pictures of chemical industries and laboratories. - Posters and charts. Instructional Resources: locally available, chemical industries. Teacher: i. Application of chemistry and adverse effect on chemicals. ii. Take students to visit chemical industries e.g. paints, tie and dye, vegetable oil, petrochemical industries. iii. Explain the scientific methods of enquiry using specific examples. Teacher: - Uses pictures of some local industries to guide students to identify chemical industries in Nigeria. - Initiates and guide discussion on the economic importance of the chemical industries. Instructional Resources: - Pictures - Charts (a) 4 (b) CHEMICAL INDUSTRIES Excursion to chemical industries 5 STANDARD SEPARATION TECHNIQUES FOR MIXTURES 1. Classification of substances 2. Filtration, Evaporation, Decantation, Floatation, Frostation. 3. Crystallization and Fractional Crystallization. 6 STANDARD SEPARATION TECHNIQUES FOR MIXTURES 1. Distillation and fractional distillation. 2. Precipitation 3. Magnetization (magnetism). 7 STANDARD SEPARATION TECHNIQUES FOR MIXTURES 1. Chromatography 2. Sublimation 3. Pure and impure substances (c) Teacher: - Takes students on field trips to chemical industries. - Guides students to observe: i. the processes going on in the industries ii. the various ways these industries degrade the environment. iii. Suggest ways of reducing environmental problems. Instructional Resources: Chemical industries in the locality Teacher: -Guides students to understand underlying principles behind the choice of a separation technique for a particular mixture. -Demonstrates the method of separation. Instructional Resources: -Water -Sand -Common salt -Filter paper -Evaporation dish Teacher: Demonstrates the method of separation Instructional Resources: - Liebing condenser - Magnets Teacher: Demonstrates the determination of melting point for solids and boiling points for liquids. Instructional Resources: - Ink - Separating funnel, - Cubes of sugar. (a) 8 (b) PARTICULAR NATURE OF MATTER 1. Physical and chemical changes 2. Atoms and molecules 3. Dalton’s Atomic theory 9. PARTICULAR NATURE OF MATTER 1. Constituents of atoms, Protons, Neutrons and electrons. 2. Arrangement of electrons around the nucleus. PARTICULAR NATURE OF MATTER 1. Atomic number, mass number and isotopy. 2. Relative atomic masses based on C14 isotope Revision Examinations Examinations 10 11 12 13 (c) Teacher: Demonstrates physical and chemical changes using simple examples like burning of candle, salts dissolved in water, burning of magnesium ribbon and preparation of pap (akamu) and starch. ii. To guide students to make chalk (CaCO3) as a chemical change. Instructional Resources: - Water - Common salt - Sugar - Candle - Matches - Models (coloured beads) - Calcium carbonate (calcium trioxocarbonate iv) [CaCO3] Teacher: To guide students to calculate the empirical formula from percentage composition. Teacher: Guide the students to calculate the relative molecular mass of a compound. Revision Examinations Examinations