observations of drosophila eggs
advertisement

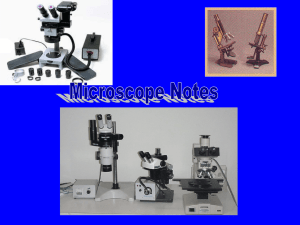
OBSERVATIONS OF DROSOPHILA EGGS Name: _________________________________ Remember to maintain your laboratory notebook entries as you conduct the laboratory activities. Otherwise, you need to do so on your own time, and before your notebook is due. Grape Juice Agar Method 1. Anesthetize the flies in your grape juice agar egg laying apparatus by placing the mesh side down on the flow buddy fly pad. Determine their gender and count each. Record the eye color and numbers in the table below and return these flies to an appropriate labeled vial of fruit flies. 2. Observed each of the prepared quadrants and tally the number of eggs in each. Agar Agar + Scoring Agar + Yeast Agar + Scoring and Yeast Eye Color & Number of Adult Males Eye Color & Number of Adult Females Number of Fruit Fly eggs 3. Record your data on the white board and copy everyone’s data into your laboratory notebook. Make specific observations regarding the egg laying, calculate and prepare a graph for your results, describe your graph in words, and make conclusions regarding egg laying in drosophila. Grape Juice Agar Eggs 1. After you have completed all the work above, prepare a clean petri dish by just filling the bottom surface with insect Ringer’s solution (a salt solution). Then, using a needle or similar implement transfer 5 eggs to the Petri dish. Observe the eggs under a dissecting microscope. Sketch the eggs, and record specific observations. If you have yet to determine the names of the egg parts, do so, label your drawing, and determine their specific functions as well. 2. Then, transfer one or more (but not all) of your eggs in a single drop of Ringer’s solution to a glass slide and observe it using a compound light microscope*. Place a small drop of Toluidine blue stain over the eggs. This stain will not fix or kill the specimen. Observe the eggs and record additional information about its structure in your laboratory notebook. 3. Transfer a separate individual egg onto another slide. Place a drop of undiluted Chlorox solution onto the egg and let stand for 5 minutes. This should dechorionate the egg. Wick away the Clorox and rinse the egg by applying a new drop of Ringer’s solution. You may need to do this a couple times to remove the Chlorox. Observe the egg using a compound light microscope, and attempt to determine its stage of the development using the pictorial reference provided. *Tips on Using a Compound Light Microscope 1. Solve your own problems by reading the directions carefully. 2. Plug in the microscope. Clean the lenses with lens paper to remove any smudges that may distort the image of your specimen. 3. Turn on the light and look to see that a beam is shining through the circular hole in the stage. If it isn’t you will notice an adjustable dimer that can be maneuvered to change the amount of light shining through the stage on the lower left hand side of the microscope. If you have a specimen already prepared on a slide it is also important that you can see that light is passing through the specimen. 4. Turn the rotating objective lenses so that the lower power (red), or shortest, lens is in line above the stage. The low power objective along with the eye piece magnifies the specimen 100X. You will also want to adjust the ring below the stage so that the line is lined up with the appropriate power. 5. Use the coarse adjustment knob (large inner knob) to move the stage as far away from the objective lenses as possible. 6. Place the slide on the stage by pull back the slide lever, slide the slide in place at the back of the slide holder, and carefully bring the lever back in place so that it holds the slide firmly. 7. Finally, peer through the eye piece and use the coarse adjustment knob to slowly bring the stage closer and closer to the low power objective lens. If you your specimen is under a cover slip, it can sometimes help to focus on the edge of the cover slip before using the slide controls to move the specimen into place. 8. Once you have focused on the specimen using the coarse adjustment knob, you can further use the fine adjustment knob (smaller outer knob) to more carefully control the focus. 9. If your specimen is small enough that higher magnification would be appropriate, carefully rotate to the medium power objective, the next largest lens in length, is positioned in line over the specimen. You shouldn’t need to adjust the coarse adjustment. As you increase in magnification, the amount of light shining through the specimen is reduce, and may need to be adjusted. This medium power objective along with the eye piece magnifies the specimen 400X. Again, you will also want to adjust the ring below the stage to the appropriate power. 10. You may use the next higher magnification is necessary, with similar caveats as mentioned for the medium power lens. The high power objective along with the eye piece magnifies the specimen 1000X. Adjust the ring under the stage again. 11. DO NOT rotate the objectives to the oil emersion lens, without being instructed on how and when to do so. This lens requires supplementary materials for its use. If it is used improperly, it can damage the lens. 12. When finished, 1) return or dispose of the slide in an appropriate manner, 2) return the objectives to their original low power position, 3) replace the cord appropriately, 4) replace the microscope cover, and 5) return the microscope to the microscope cabinet.