20140703031008!LDCP_..
advertisement

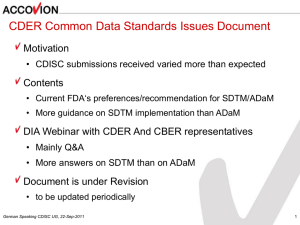
Legacy Data Conversion Plan <Sponsor Name> <Compound Name> Version 2016-02-08xx-xx <Compound Name> Legacy Data Conversion Plan Legacy Data Conversion Plan Contents 1. Introduction........................................................................................................................................... 3 1.1 Purpose.......................................................................................................................................... 3 1.2 Acronyms ...................................................................................................................................... 3 1.3 Study Data Standards and Dictionary Inventory ......................... Error! Bookmark not defined. 2. Protocol Description ........................................................................... Error! Bookmark not defined. 2.1 Protocol Number and Title.......................................................... Error! Bookmark not defined. 2.2 Protocol Design ........................................................................... Error! Bookmark not defined. 2.3 Trial Design Datasets .................................................................. Error! Bookmark not defined. 2.3.1. TA – Trial Arms.................................................................. Error! Bookmark not defined. 2.3.2. TE – Trial Elements ............................................................ Error! Bookmark not defined. 2.3.3. TV – Trial Visits ................................................................. Error! Bookmark not defined. 2.3.4. TI – Trial Inclusion/Exclusion Criteria ............................... Error! Bookmark not defined. 2.3.5. TS – Trial Summary ............................................................ Error! Bookmark not defined. 3. Subject Data Description .................................................................... Error! Bookmark not defined. 3.1 Overview ..................................................................................... Error! Bookmark not defined. 3.2 Annotated CRFs .......................................................................... Error! Bookmark not defined. 3.3 SDTM Subject Domains ............................................................. Error! Bookmark not defined. 3.3.1. AE – Adverse Events .......................................................... Error! Bookmark not defined. 3.3.2. DS – Disposition ................................................................. Error! Bookmark not defined. 3.3.3. EX – Exposure .................................................................... Error! Bookmark not defined. 3.3.4. Dataset – Dataset Label....................................................... Error! Bookmark not defined. 4. Data Conformance Summary.............................................................. Error! Bookmark not defined. 4.1 Conformance Inputs .................................................................... Error! Bookmark not defined. 4.2 Issues Summary .......................................................................... Error! Bookmark not defined. 4.3 Additional Conformance Details ................................................ Error! Bookmark not defined. Appendix I: Inclusion/Exclusion Criteria ..................................................................................................... 6 Appendix II: Conformance Issues Details .................................................................................................... 7 PDF created 2016-02-08 Page 2 of 7 <Compound Name> Legacy Data Conversion Plan 1. Introduction 1.1 Purpose This document provides context for the conversion of raw data to standardized data for <submission, pre-NDA>. In addition, this document summarizes the conversion results along with any issues encountered and the resolution of the issues. Any outstanding issues will also be summarized. 1.2 Acronyms Acronym Translation 2. Legacy Data Summary Identify studies included and phase Assumptions on the data – such as, could only convert some of the data Was data converted from actual datasets or did it have to be entered manually from the CRF into the data entry system? Did a vendor manually enter the data? Any translations from non-English to English? (Protocol, CSR, data) Type of validation run on the legacy data, particularly on data that had to be entered manually (such as double data entry, controls, etc.) Were all SDTM domains created or a subset such as safety or efficacy? Was all data converted? Phase I sometimes only requires that a subset of the data is submitted. Was this done? For example, for the following studies only the following domains were created: AE, EX, etc. Point to where more information can be found (such as SDSP, SDRG) 3. Converted Data Summary Include text on the process for how the data was converted, including version of SDTM. Data converted that could affect CSR, such as race. Could include diagram as well as text. PDF created 2016-02-08 Page 3 of 7 <Compound Name> Legacy Data Conversion Plan Mention if upcode data, such as MedDRA version or WhoDRUG version plus other dictionaries if applicable. Refer to SDRG for more information. Dictionaries used, such as converting lab test codes to text. Mention if saved raw data in supplemental domains. How was the SDTM data validated aside from Open CDISC? Were reports run to ensure numbers match the CSR from the analysis datasets that were created by the sponsor? Was raw data submitted or bridging document created? Is ADaM created from the SDTM or were these ‘dead end’ conversions? Was analysis rederived based on the SDTM? Was controlled terminology followed from legacy to SDTM? Was it updated to a particular version of controlled terminology? What type of data standards were used in addition to controlled terminology? For example, if there are 1’s and 0’s used for Yes and No. Body systems, too. Didn’t match current SOC but had to rename in SDTM. 4. Conversion Results If common theme, provide it here. For example, if certain trial domains were not created or if SDTM domains could not be fully populated. Or if certain SDTM required variables, such as EPOCH, could not be populated. Refer to SDRG for more details. Table with old variable name, SDTM name, comments (include data collected but not converted). Sounds like a bridging document from raw data to SDTM. If don’t send raw data, is this meaningful? Refer to SDRG for open CDISC issue summary. Look at .2 of Mario’s example. Issues Encountered and Resolved Issue Resolution Comments Outstanding Issues Issue PDF created 2016-02-08 Comments Page 4 of 7 <Compound Name> Issue PDF created 2016-02-08 Legacy Data Conversion Plan Comments Page 5 of 7 Study Data Reviewer’s Guide <Compound Name> Appendix I: Conversion Matrix CRF Page (?) Raw Dataset Standard or Dictionary SDTM Domain Comments Version SDTM Controlled Terminology Medications Dictionary Medical Events Dictionary Other standards (optional) PDF created 2016-02-08 Page 6 of 7 <Compound Name> Study Data Reviewer’s Guide Appendix II: Issues Details (Text here) PDF created 2016-02-08 Page 7 of 7