Chapter 2 - Winona State University
advertisement

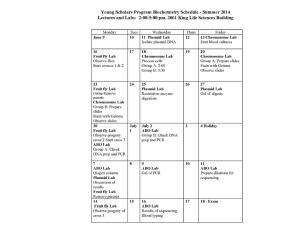
Chapter 2 Review Questions 1. What type of monomer is polymerized to form DNA? What type of monomer is polymerized to form RNA? 2. What are the three components are necessary to form a nucleotide? What must be left off to form a nucleoside? 3. Below are the pictures of a nucleotide. Please label the carbons. Which carbon is the phosphate group bound to? Which carbon is the nitrogenous base bound to? Also for each nucleotide state which sugar is present, and which nitrogenous base is present. Please name this nucleotide. 4. Please state the nitrogenous bases common to DNA. Which are purines and which are pyrimadines? 5. Please state the nitrogenous bases common to RNA. Which are purines and which are pyrimadines? 6. Please state Chargaff’s rules. Please explain in detail why these rules apply? 7. Please explain the difference between deoxyribose and ribose specifically. Also, please discuss how this effects each structure and chemistry. 8. Please give the appropriate name for the nucleotide/nucleoside based off each of the descriptions below. a. Contains deoxyribose and guanine b. Contains deoxyribose, adenine and two phosphates c. Contains ribose, uracil and three phosphates d. Contains ribose, cytosine and one phosphate e. Contains deoxyribose and thymine 9. For the nucleotide below, please appropriately label the phosphates. 10. What type of reaction allows for the joining of two nucleotides to allow for elongation of a DNA strand? Please state the reason for the name of the reaction that allows for the joining of two nucleotides. What type of bond is formed in this reaction. Please discuss which groups are involved in the reaction and which carbons they are bound to on the pentose sugar. 11. Does a single DNA strand have a specific polarity? Please explain your answer in detail. 12. Please take the sequence of the DNA strand below (not written in proper conventions), and write the sequence of its complement. When you write the sequence, you must use the proper conventions. 5’ ACCTCGTTGGTATTCTTATCA 3’ 13. Please state the two forces that are necessary to allow for the structural integrity of a double stranded molecule of DNA. Please describe each force. 14. How many hydrogen bonds are present between an A-T base pair 15. How many hydrogen bonds are present between a G-C base pair? 16. Which base pair is stronger? Please explain why. 17. What is meant by Watson-Crick base pairing? Please name a non-WatsonCrick base pair that may be found in DNA. 18. When looking at the exterior of a DNA molecule, name two different features you may see. Please describe them for the B-type DNA double helix. 19. Please state three different forms that the DNA double helix can take. Please describe each, and state which conditions are necessary for each to form? 20. Which forms of DNA double helices are physiologically relevant. Please explain your answer. 21. Please state one method by which you could denature double stranded DNA? How does this method denature the DNA? 22. Please state how much of each nucleic acid you would have if you received an A260 of 1. a. RNA b. ssDNA c. dsDNA 23. You take your original tube of double stranded DNA. You make a 1:20 dilution of your sample and then measure the A260. Your A260 = 0.56. Please calculate the concentration in your original tube. 24. You take your original tube of RNA. You make a 1:50 dilution of your sample and then measure the A260. You A260 = 1.12. Please calculate the concentration in your original tube.