Microcirculation endothelial cell cycling is selectively increased in
advertisement

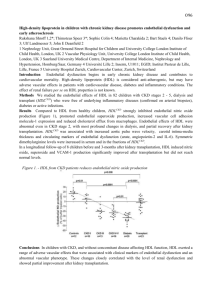
Microcirculation endothelial cell cycling is selectively increased in antibodymediated rejection of kidney transplants, but not in other diseases S Osasan1,2, D de Freitas 2,J Sellares2, J Chang2, L Hidalgo1,2, M Mengel1,2, P F Halloran2, B Sis1,2 1 Department of Laboratory Medicine and Pathology, 2Alberta Transplant Applied Genomics Centre, University of Alberta, Edmonton, AB, Canada. Background: Antibody-mediated rejection (ABMR) is dominated by microcirculation inflammation and endothelial injury, caused by donor specific antibodies. Objective: We hypothesized that ABMR is associated with a greater endothelial repair response compared to other diseases operating in kidney transplants. Method: We related microcirculation endothelial cell cycling to histopathology lesions, diagnoses, and whole-genome microarrays in 100 kidney transplant biopsies and 40 normal implantation biopsies. We performed double immunostaining for Ki-67 and CD31 to identify capillaries with cycling endothelial cells. We quantified the microcirculation endothelial cell cycling by counting number of Ki-67+CD31+ glomerular and peritubular capillaries in the entire cortical area of the biopsies. Results: The transplant biopsies showed higher numbers of capillaries with cycling endothelial cells in comparison to normal controls (p=0.003). The mean microcirculation endothelial cell cycling was highest in ABMR compared to other diseases. (figure1) Increased microcirculation endothelial cell cycling correlated with donor specific HLA antibody (p=0.003), microcirculation lesions (glomerulitis, capillaritis, transplant glomerulopathy), and C4d staining (p<0.05). Furthermore, transcript sets representing the molecular burden of active ABMR (endothelial cell, NK-cell, and macrophageassociated transcripts as well as interferon gamma regulated transcripts) correlated with increased microcirculation endothelial cell cycling (p<0.05). Within the ABMR biopsies, increased microcirculation endothelial cell cycling correlated with higher grades of glomerulitis (p=0.01) and peritubular capillaritis (p=0.08). Interestingly, cycling was not increased in TCMR, suggesting that T cells cross the microcirculation in TCMR without damaging the capillaries. Conclusion: We conclude that endothelial repair response is selectively increased in kidneys with ABMR, reflecting the burden of active microcirculation injury. p=0.001 p=0.021 Microcirculation endothelial cell cycling p=0.017 p=0.004 3 2 1 0 n Controls n = 40 ABMR n = 25 TCMR n = 11 Borderline n = 18 GN n = 12 Transplant biopsies = = = TCMR = GN = Controls = Mixed ABMR & TCMR C4d+ ABMR C4d- ABMR T Cell-Mediated Rejection Glomerulonephritis 20 living and 20 deceased donor implant biopsies OTHER n = 34