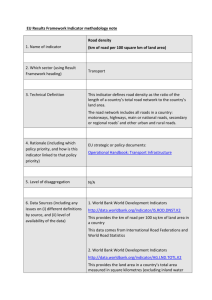
pH = pK a + log
advertisement

…………اسرة الفرسان.. pharmaza ………. ………… اسرة الفرسان.. pharmaza …… ………… اسرة الفرسان.. pharmaza 1. Acid – base 1. Introduction (lecture 1) Electrolyte and the theory of electrolytic Dissociation: Electrolytes: allow the electric current to pass (Conduct the electricity) e.g mineral acid, caustic alkalies and salts. non–electrolytes : not allow the current to pass, e.g : glycerin and ethyl acetate and cane sugar. "ionisation" or "dissociation“: when electrolyte is dissolved in water it decomposes by the passage of the electric current into its components "ions“ at the cathode and the anode. Degree of dissociation 𝜕= 𝑁𝑜 𝑜𝑓 𝑚𝑜𝑙𝑒𝑐𝑢𝑙𝑒 𝑑𝑖𝑠𝑠𝑜𝑐𝑖𝑎𝑡𝑒 𝑡𝑜𝑡𝑎𝑙 𝑁𝑜 𝑜𝑓 𝑚𝑜𝑙𝑒𝑐𝑢𝑙𝑒 𝑏𝑒𝑓𝑜𝑟𝑒 𝑑𝑖𝑠𝑠𝑜𝑐𝑖𝑎𝑡𝑖𝑜𝑛 Strong electrolyte: “a” near unit i.e completely dissociate Strong acid as HCl, H2SO4, HNO3 . Weak acid as CH3COOH (acetic acid) Weak electrolyte: the value of “a” far from unit Strog base as NaOH, Ba(OH)2 Weak base asNH4OH Law of mass action "The rate of a chemical reaction is proportional to the active masses of the reacting substances." In a reaction: A + B ⇋ C + D K [C][D] [A]][B] K "equilibrium constant" The dissociation of water H2O ⇌ H+ + OH– [H+] . [OH] According to the law of mass action: = Kw K "The ionic product of water" = = 1014 [H+]2 = Kw = 1 x 1014 [H+ ] 10 -14 10 7 1 [H+ ][OH - ] [H2O] …………اسرة الفرسان.. pharmaza ………. ………… اسرة الفرسان.. pharmaza …… ………… اسرة الفرسان.. pharmaza Hydrogen ion exponent (pH): equal to the logarithm of the hydrogen ion concentration with a negative sign pH = –log [H+] pOH = –log [OH–] pKw = –log Kw pKw = pH + pOH pH = pKw – pOH or pH = 14 – pOH or pOH = 14 – pH Lecture 2 2. Acid–base equilibrium pH calculations 1. Solution of strong acids and strong bases 1) Calculate the pH value of a solution of a completely ionised 1.0 N solution of acid; or base. ? [H+] = 1M pH = –log 1 = 0 (zero) similarly, in a completely ionised 1.0 N solution of base [OH–] = 1 M pOH = –log 1 = 0 (zero) 2) Calculate the [H+] hydrochloric acid? [H+] = 0.009 N and pH of 0.009 N pH = – log 0.009 = 2.05 3) Calculate the pH values of a solution of sodium hydroxide whose [OH –] is 1.05 x 10–3? pOH = – (log 1.05 x 10–3) = 2.98 2 …………اسرة الفرسان.. pharmaza ………. ………… اسرة الفرسان.. pharmaza …… ………… اسرة الفرسان.. pharmaza pH = 14 – 2.98 = 11.02 4) Calculate the hydrogen ion concentration of a solution of pH 5.3? pH = – log [H+] 5.3 = –log [H+] [H+] = antilog – 5.3 =10-5.3 =5.01 x 10–6 M 5) Calculate the hydroxyl ion concentration of a solution of pH 10.75? pOH = 14 – 10.75 = 3.25 [OH–] = the antilog of –3.25 =10-3.25 = 5.62 x 10–4M weak acids e.g. CH3COOH weak bases e.g. NH4OH Salts of strong acids or bases Salts of weak acids and strong bases e.g Sodium acetate H2O + CH3COO ⇌ CH3COOH + OH Salts of weak bases and strong acids: e.g ammonium chloride; NH4+ + H2O ⇌ NH4OH + H+ pH = ½ (pKa + pCa) pH = pKw – ½ (pKb + pCb) pH = 7 Salts of weak acids and weak bases: ammonium acetate pH = ½ pKw + ½ pKa – ½pKb pH = ½ (pKw – pCs + pKa) pH = ½ (pKw + pCs – pKb) degree of hydrolysis h= y/Cs percentage hydrolsis is: 100 x (y/Cs) 6) Calculate the pH and [H+] of 0.10 N acetic acid (pKa= 4.76)? pH = ½ (pKa + pCa) = ½ x 4.76 + ½ (– log 10–1) = 2.38 + 0.5 = 2.88 7) Calculate the pH and [H+] of a 0.0045 M solution of phenobarbital (pKa= 7.41)? PCa = – log (4.5 x 10–3) = 2.35 pH = ½ (7.41 + 2.35) = 4.88 + -4.88 [H ] = antilog – 4.88 = 10 = 1.32 x 10–5 M 8) Calculate the pH, and the [H+] of 0.13 N ammonia solution (pKb = 4.76)? 3 …………اسرة الفرسان.. pharmaza ………. ………… اسرة الفرسان.. pharmaza …… ………… اسرة الفرسان.. pharmaza pH = 14 – ½ (4.76) – ½ (– log 0.13) = 14 – ½ (4.76 + 0.89) = 11.18 [H+] = antilog – 11.18= 10-11.18 = 6.6 x 10–12 M 9) Calculate the pH and the [H+] of a 0.0037M solution of cocaine base (pKb = 5.59)? pH = 14.00 – ½ (5.59 + 2.43) = 9.99 [H+] = antilog of – 9.99 = 10-9.99 = 1.02 x 10–10 10) Calculate the pH, the [H+], the [OH–], and the degree of hydrolysis of a 0.1 M solution acetate (pKa = 4.76)? pH = ½ (14.00 – 1.00 + 4.76) = 8.88 [H+] = antilog – 8.88 = 10-8.88 = 1.3 x 10–9 M [OH+] = 10–14/ [H +] = 10-14/ 1.3x10–9 = 7.5x10–6 M h= 7.5x10 6 = 7.5 x 10–5 0.1 %h 7.5x10 6 100x(7.5x10 5 ) 0.007% hydrolysed 0.1 11) Calculate the pH of a 0.165 M solution of sodium sulphathiazole (salt of weak acid and steong base) (pKa = 7.12)? pCs = – log 0-165 = 0.78 pH = ½ (14.00 – 0.78 + 7.12) = 10.17 12) Calculate the pH, the [H+] and the degree of hydrolysis of a 0.05 N solution of ammonium chloride. pH = ½ (14.00 + 1.30 – 4.76) = 5.27 [H+] = 5.4 x 10–6 M 6 Degree of hydrolysis = 5.4x10 1.1x10 4 0.05 Percentage of hydrolysis = 100 x (1.1 x 10–4) = 0.011 % 13) Calculate the pH of a 0.025 M solution of ephedrine sulphate (pKb = 4.64)? pCs = – log 2.5 x 10–2 = 1.58 pH = ½ (14.00 – 1.58 – 4.64) = 5.47 4 …………اسرة الفرسان.. pharmaza ………. ………… اسرة الفرسان.. pharmaza …… ………… اسرة الفرسان.. pharmaza 2. Buffer solutions (lecture 4) Solution resists changes in pH caused by addition of small amounts of acid or base; or upon dilution Acidic buffer Weak acid [HA] and its salt [A] Basic buffer Weak base [BOH] and its salt [B+] CH3COOH ⇌ CH3COO- + H+ CH3COONa ⇌ CH3COO- + Na+ NH4OH ⇌ NH4+ + OHNH4Cl ⇌ NH4+ + Cl- Henderson equations pH = pKa [B ] [BOH] [Salt] pH = pKw – pKb – log [Base] [A ] + log [HA] pH = pKa + log pH = pKw – pKb – log [Salt] [Acid] 14) Calculate the pH of a solution containing 0.10 N acetic acid and 0.10 N sodium acetate? According to Henderson equation: pH = 4.76 + log 0.10 4.76 0.10 If the pH of this solution is compared with the pH of a solution which contains 0.10 N acetic acid only; then pH = ½ (pKa + pCa) = ½ (4.76 + 1) = 2.88 It is seen that the pH of acetic acid solution has been increased almost 2 pH units; i.e. the acidity has been reduced to about one – hundredth of its original value by the presence of an equal concentration of a salt with common ion. 15) Calculate the pH of the solution produced by adding 10.0 ml of N HCl to 1 liter of solution which is 0.1 N in acetic acid and 0.1 N in sodium acetate (Ka= 1.82 x 10-5) 5 …………اسرة الفرسان.. pharmaza ………. ………… اسرة الفرسان.. pharmaza …… ………… اسرة الفرسان.. pharmaza pH = pKa + log [Salt] [Acid] Neglecting the volume change from 1000 to 1010 ml, the HCl reacts with acetate ion forming practically undissociated acetic acid. H+ + CH3COO ⇌ CH3COOH [CH3COO] = 0.1 – 0.01 = 0.09 [CH3COOH]= 0.1 +0.01 = 0.11 pH = 4.74 + log 0.09 = 4.74 – 0.09 = 4.65 0.11 Hence the addition of strong acid, the pH change only by 4.74 – 4.65 = 0.09 pH unit, whereas, if 10 ml of N-hydrochloric acid were added to liter of pure water (pH =7), the pH would have changed from 7 to – log (0.01) = 2, i.e by 5 pH units. This illustrates the action of the acetic acid – sodium acetate buffer 16) Calculate the pH of a solution containing 0.07 N ammonia, and 0.28 N ammonium chloride? pH = 14.00 – 4.75 – log 0.28 = 8.64 0.07 If the pH of this solution s compared with the pH of a solution which contains 0.07N ammonia only; being: pH = 14.00 - ½(4.76 + 1.16) = 11.04 Therefore, the addition of the ammonium chloride has decreased the ionisation of the base such that the pH was decreased from 11.04 to 8.64. Properties of buffer mixtures: 1. Effect of temperature on pH: acidic buffer : slight or no effect basic buffers temperature has marked effect on pH of basic buffer (owing to Kw which appears in the equation) 2. Effect of dilution: dilution should have no effect on pH (from Henderson equation concentrations are a ratio) 6 …………اسرة الفرسان.. pharmaza ………. ………… اسرة الفرسان.. pharmaza …… ………… اسرة الفرسان.. pharmaza Buffer Capacity The buffer capacity is defined as the number gram equivalent of strong acid or strong base required to change the pH of one liter of buffer solution by one unit. 17) Calculate the buffering capacity of a solution containing 0.1 gm equivalent of sodium acetate and acetic acid? PKa = 4.76 pH = 4.76 + log 0.1 = 4.76 0.1 To increase (or decrease) the pH by one unit, the ratio salt/acid will have to alter by a factor of 10: 5.76 = 4.76 + log log [salt] [acid] [salt] [salt] = 1; therefore = 10 [acid] [acid] To reach pH 5.76, then [salt] will have to increase to a concentration of about 0.182-gram equivalent, and the [acid] to decrease to about 0.018. The buffering capacity of the buffer mixture is therefore; 0.100–0.082 gram equivalent towards strong base, because the decrease in acid is brought about by adding 0.082 gram equivalent of strong base. 3. Acid-Base indicators (lecture 5) Organic dyes change their color depend on the change of the pH Basic indicator InOH ⇌ OH- + In+ e.g methyl orange Acidic indicator Hln ⇌ H+ + In- (1) e.g phenolphthalein (2) Theories of acid base indicator: Ostwald Theory: If the acid indicator is added to an acidic solution, the concentration of the hydrogen ion term on the right-hand side of equation (1) is increased and the ionization of the indicator is repressed by the common ion effect. 7 …………اسرة الفرسان.. pharmaza ………. ………… اسرة الفرسان.. pharmaza …… ………… اسرة الفرسان.. pharmaza The indicator is then predominantly in the unionised form of HIn, the acid colour. If on the other hand, the indicator is added to a basic solution, the [H+] is reduced by reaction of the acid indicator with the base, and reaction (1) proceeds to the right yielding more ionised indicator [In-] and the basic colour predominates. The reverse is true for basic indicators; in basic solution, the reaction (2) proceeds to the left and the basic colour is prominent. Objectives of Ostwald theory: 1. Phenolphthalein indicator has red colour in slightly alkaline solution, when more alkali is added give colourless, while the expected from the theory, the colour should be increased. 2. Slow colour change in some indicators, while ionic reactions are usually instantaneous. 3. Some indicators show their colour changes in non aqueous media where ionisation is markedly decreased. Basic indicator Acid indicator HIn HIn" pH = pKind H+ + OHIn OHIn" In''- + OHand pOH = pKind – log [OH In] In"– [In " ] " + log [In ] [HIn] pH = pKind + log " pH = pKw – pKind – log [In ] [OH In] [basic colour] [acid colour] pH = pKw – pKind – log [acid colour] [basic colour] Transition range of acid – base indicators The first change in the acid colour of an indicator becomes visible when the ratio (basic colour) /(acid colour) becomes 1/10 1 pH = pKind + log = pKind –1 10 and the eye cannot detect a change in the basic colour of the indicator until the ratio (basic colour)/(acid colour) has become 10 /1 10 pH = pKind + log = pKind +1 1 Effective transition range of the indicator, and may be expressed as follows: pH = pKind. 1 8 …………اسرة الفرسان.. pharmaza ………. ………… اسرة الفرسان.. pharmaza …… ………… اسرة الفرسان.. pharmaza 1) Phenolphthalein is one color indicator (example of acid indicator) colorless pink colorless pH ≤ 8 pH 8 – 10 pH ≥ 10 Benzenoid structure Quinonoid structure Tribasic salt 2) Methyl orange in two colour indicator (example of basic indicator Yellow (azo – structure) Red pH ≥ 4.4 pH ≤3.1 Mixed indicators: It’s a mixture of two indicators, to produce a definite and characteristic colour change within a very narrow range of pH. An example of such mixed indicator is bromocresol green and methyl red; the acidic and basic colours of the mixture are orange and green respectively. Screened indicators: Screened indicator increase the sharpness of the colour change at the end point of. a titration. A screened indicator is a mixture of an indicator and an inert dye whose colour does not change with pH. An example is the so-called "modified methyl orange” is a mixture of methyl orange with the inert dye xylenecyanol F.I. This screened indicator is purple-red in acid medium and green in alkaline medium, and gray at its intermediate point. Turbidity indicators: Turbidity indicators are salts of weak organic acids or bases of high molecular weight, which coagulate and settle out of the solution at a definite pH value. 9 …………اسرة الفرسان.. pharmaza ………. ………… اسرة الفرسان.. pharmaza …… ………… اسرة الفرسان.. pharmaza Universal of multirange indicators: By suitable mixing of several indicators, the colour change made to extend over a considerable portion of the pH range. They are not suitable for titration but indicate roughly the pH of the solution (approximate determination of pH) e.g. a mixed indicators containing thymol blue, methyl red, bromothymol blue and ph.ph. give the following colors at various pH values: pH 2 4 6 8 10 colour red yellow blue orange green 4. Neutralization curves (lecture 6) The curve obtained by plotting pH against ml of titrant. 1- Strong acid versus strong base: (hydrochloric acid with sodium hydroxide) Both the titrant and the analyte are completely ionized. H+ + Cl- + Na+ + OH - →H2O + Na+ + ClTo titration curve for 100 ml of 1 M HCl with 1 M sodium hydroxide solution. Calculation of pH during titration: a) At the beginning pH= -log [H+] = - log 1 = zero of titration: b) During the For 50 ml of base 1 titration: [H+] = 50 x = 0.333 or pH=0.48 150 For 75 ml of base 1 [H+] = 25.x 175 = 0. 143 or pH = 0.85 For 90 ml of base + [H ] = 10 x 1 = 0.0527 190 or pH = 1.28 For 99 ml of base 1 [H+] = 1x199=5.02x l0-3 or pH = 2.30 For 99.9 ml of base + C) At the equivalence point: 1 [H ] = 0.1 x 199.9= 5.02x1 0-4 or pH = 3.30 The solution contains only NaCI and water The pH value of the solution is 7.00 10 …………اسرة الفرسان.. pharmaza ………. ………… اسرة الفرسان.. pharmaza …… ………… اسرة الفرسان.. pharmaza c) Beyond the equivalence point: The solution contains excess alkali: With 100.1 ml base [OH-] 0.1 = 200.1=5.00x10-4 or pOH 3.3 and pH =10.7 With 101 ml base [OH ] = 1/201 =5.00x10-3 or pOH 2.3 and pH =11.7 The appropriate indicator is one that changes colour between pH 33 and pH l0.5. Phenolphthalein methyl red and methyl orange are most often used. - Indicators 2. Titration curves of Strong bases versus Strong acids: Analogous in calculation the previous curve (invert) 11 …………اسرة الفرسان.. pharmaza ………. ………… اسرة الفرسان.. pharmaza …… ………… اسرة الفرسان.. pharmaza 3. Titration curves of weak acids versus strong bases: Consider the titration of 100.0 ml. of 0.10 N acetic acid (pKa = 4.76) with 0.10 N sodium hydroxide. HAc + Na+ + OH - →H2O + Na+ + ACa) pH before adding The pH of acetic is calculated using equation titrant: pH = ½ (pKa + Ca) pH = ½ (4.76 + 1.00) = 2.88 b) pH during titration: The additions of base procedures a buffer of acetic acid and sodium acetate. The pH of the solution is calculated from the Henderson equation. pH = pKa + log [Salt] [Acid] Thus, on adding 25 ml. of base, the molar concentrations of the two components of the buffer will be: [acid] = 75 x 0.1 125 [salt] = 25 x pH = 4.76 + log c) pH at the equivalent Point: on adding 50 ml. of base, the concentrations of acid and salt are equal ; so that the log term in the Henderson equation =0 , and the pH = 4.76. the acetic acid is quantitatively converted to sodium acetate whose molar concentration is: [NaAc] = d) pH beyond equivalence point: 75 = 4.26 25 0.1 125 100 x 0.1 200 The pH of the solution is calculated from equation pH = ½ (pKw – pCs + pKa) pH = ½ (14.00 – 1.30 + 4.76) = 8.73 On adding 50.1 ml. of base: [NaOH] = [OH–] = pOH = 4.3 0.1 x 0.1 200.1 = 5.0 x 10–5 pH = 14.00 – 4.30 = 9.7 12 …………اسرة الفرسان.. pharmaza ………. ………… اسرة الفرسان.. pharmaza …… ………… اسرة الفرسان.. pharmaza Indicators methyl orange cannot be used because its transition range does not fall within the vertical part of the curves. A suitable indicator should be one whose transition range exists in the basic region of the pH scale, e.g. phenolphthalein. 4. Titration curves of weak bases versus strong acids: analogous to that described for the case of weak acids and strong bases, except that methyl orange is used as indicator. E.g. titration of ammonia against HCl) 13 …………اسرة الفرسان.. pharmaza ………. ………… اسرة الفرسان.. pharmaza …… ………… اسرة الفرسان.. pharmaza Application of Neutralization Titrations (lecture 7) 1. Determination of ammonium salts (e.g. NH4Cl) 1. Formol titration: Formaldehyde + ammonium salt = hexamethylene tetramine + an amount of acid equivalent to the ammonium salt. The liberated acid can be titrated against standard alkali using phenolphthalein (ph.ph.) as indicator. 4NH4Cl + 6HCHO → (CH2)6N4 + 4HCl + 6 H2O 2. Direct method: Ammonium salt + sodium hydroxide and the mixture distilled. Ammonia is quantitatively expelled, and is absorbed in an excess standard acid. The excess of acid is back titrated in the presence of methyl-orange as indicator. NH4+ + OH− → NH3↑ + H2O NH3 + HCl → NH4Cl The ammonium salt is boiled with known excess of standard sodium hydroxide solution. The bailing is continued until no more ammonia is evolved. The excess of sodium hydroxide is titrated with standard acid, using methyl orange as indicator. 3. The indirect method 2. Determination of Carbonate and Bicarbonate in Mixture The analysis of such mixture requires two titrations, one with an alkaline-range indicator, such as ph.ph. and the other with an acid-range indicator, such as methyl-orange. Na2CO3 + HCl → NaHCO3 + NaCl . . . . . . . . . pH 8.3 MaHCO3 + HCl → CO2 + H2O + NaCl . . . . . . pH 3.8 A portion of cold solution is slowly titrated with standard hydrochloric acid using ph.ph. as indicator. This volume of acid (V1) corresponds to half the carbonate: CO32+ H+ → HCO3Another sample of equal volume is then titrated with the same standard acid using methylorange, as indicator. The volume of acid (V2) corresponds to carbonate + bicarbonate; hence 2 V4 = carbonate and V2 - 2Vi = bicarbonate. 14 …………اسرة الفرسان.. pharmaza ………. ………… اسرة الفرسان.. pharmaza …… ………… اسرة الفرسان.. pharmaza 3. Determination of a Mixture of Carbonate and Hydroxide (analysis of commercial caustic soda) (Must be in cold (as near 0°C), and loss of carbon dioxide must be prevented as far as possible by keeping the tip of the burette immersed in) Method 1: Method 2: (two indicators method) First portion: Total alkali (carbonate + hydroxide) is determined by titration with standard acid, using methyl orange as indicator. E.p. 1 Second portion: For hydroxide content only, carbonate is precipitated with a slight excess of barium chloride solution, and, without filtering, the solution is titrated with standard acid using phenolphthalein as indicator. E.p. 2 Na2CO3+ BaCl2 = BaCO3 (insoluble) + 2 NaCl e.p. 1 – e.p 2 = carbonate First portion : For OH- and ½ CO32-(to HCO2-) The solution titrated with acid using a mixed indicator (composed of thymol blue and cresol red) till rose color = e.p 1 OH− +H+ = H2O CO32- + H+ = HCO3− Second portion For total alkalinity (all Carbonate and Hydroxide) titration with acid using methyl orange as indicator = e.p. 2 OH− +H+ = H2O CO32- + H+ = HCO3− H2CO3 = H2O + CO2 2Then CO3 = 2x (e.p2 – e.p. 1) & OH- = e.p.2 – CO32- 15 …………اسرة الفرسان.. pharmaza ………. ………… اسرة الفرسان.. pharmaza …… ………… اسرة الفرسان.. pharmaza 4. Determination of Boric Acid Boric acid acts as a weak monoprotic acid (Ka= 6.4x10-10), it cannot therefore titrated accurately with standard alkali. However, by the addition of certain organic polyhydroxy compounds, such as glycerol, mannitol, sorbitol, or glucose, it acts as a much strong monobasic and can be directly titrated with sodium hydroxide; using phenolphthalein as indicator. NaOH + H3BO3 = NaBO2 + 2 H2O 5. Determination of Borax (hydrolysable salt) When borax is dissolved in water, it is hydrolysed into: Na2B4O7 + 7 H2O = 4 H3BO3 + 2 NaOH Acid reading: solution is titrated with standard hydrochloric acid using methyl orange as indicator, it is the NaOH that is actually titrated; boric acid being of no effect on the indicator, and net reaction is: Na2B 4O7 5 H2O + 2 HCl = 4 H3BO3 + 2 NaCl Na2B 4O7 10 H2O = 2 HCl Alkali reading: The residual solution can be titrated for the remaining boric acid with standard sodium hydroxide after adding glycerol and using phenolphthalein as indicator. The reaction would be: Na2B4O7 + 5 H2O + 2 HCl = 4 H3BO3 + 2 NaCl Na2B4O7.10 H2O = 4 NaOH double the volume of standard acid. 16 …………اسرة الفرسان.. pharmaza ………. ………… اسرة الفرسان.. pharmaza …… ………… اسرة الفرسان.. pharmaza 6. Determination of Mixture of Boric Acid and Borax Solutions of alkali borates may be titrated with standard acid (e.g. HCl) using methyl orange as indicator. Na2B4O7.10 H2O + 2 HCl = 4 H3BO3 + 2 NaCl + 5 H2O While the liberated boric acid consumes 4 molecules of NaOH when titrated with alkali (e.g. NaOH), using phenolphthalein as indicator in presence of glycerol. 7. Determination of Nitrogen by Kjeldahl's Method For nitrogen (examples include amino acids, proteins, synthetic drugs, fertilizers, explosives soils). The sample is decomposed in hot, concentrated sulphuric acid to convert the bound nitrogen to ammonium ion. The liberated ammonia is distilled, collected in an acidic solution, and determined by neutralization titration. 2SO4 CaHbNc H a CO2 1 2 b H2O c NH4HSO4 - c NH4HSO4 OH CNH3 c SO32- CNH3 (c d) HCl c NH4 Cl d HCl 8. Determination of Amino Acids Amino acids are amphoteric substances that contain both acidic and basic group (i.e., they can act as acids or bases). The acid group is a carboxylic acid group (-COOH), and the basic group is an amine group (-NH2). In aqueous solutions, these substances tend to undergo internal proton transfer from the carboxylic acid group to the ammo group because the RNH2 is a stronger base than RCO2−. The result via a Zwitter ion. Since they are amphoteric, these substances can be titrated in aqueous solutions. We can consider the conjugate acid of the Zwitter ion as a diprotic acid which ionizes stepwise. 17 …………اسرة الفرسان.. pharmaza ………. ………… اسرة الفرسان.. pharmaza …… ………… اسرة الفرسان.. pharmaza 9. Biphasic Titrations For water-soluble salts the acid of which is insoluble in water, but soluble in an immiscible solvent. For example, sodium salicylate or sodium benzoate its determination by the two phase titration method; Since the salicylic acid liberated during the titration is a sufficiently strong acid to give a pH which will be "acid" to any usual indicator, this acid has to be removed as it is liberated. The ratio of solubility of salicylic acid in ether to that in water is 250:1, If the volume of ether is double or triple that of water, then the salicylic acid remaining in the aqueous phase will be quite negligible. 10. Double Indicator Titration These are direct titrations in which a mixture of two monobasic acids are titrated using two indicators, if the difference in the ionisation constants of the two acids is at least 104. Thus, it is possible to titrate hydrochloric acid in presence of acetic acid (ka= 1.8x10-5). The hydrogen ion of the hydrochloric acid suppresses the ionisation of the other weak acid by common ion effect, so that the sodium hydroxide added neutralises the hydrochloric acid first. When this reaction is 18 …………اسرة الفرسان.. pharmaza ………. ………… اسرة الفرسان.. pharmaza …… ………… اسرة الفرسان.. pharmaza complete, the pH rises to that of the other weaker acid solution which is then titrated. The pH change at the first equivalence point is not very great, and the end point determined by a colour indicator. And the second determined potentiometrically 11. Residual (or Back) Titrations These consist in the addition of a known excess of standard solution to a weighed amount of the sample and after the reaction is complete, the residual quantity of the added standard is determined. In general this method is used for: a) Volatile substances, such as ammonia. b) Insoluble substances, such as calcium oxide and calcium carbonate. Aspirin.. c) Substances which require heating with the standard reagent during the determination in which decomposition or loss of the reactants or products would occur in the process e.g lactic acid A) Determination of Mixture of Calcium Oxide and Calcium Carbonate The estimation is based on: a) CaO is more soluble in sucrose solution than in water, the complex calcium saccharate being equally alkaline as CaO. It can be titrated with standard acid (e.g. HCl). b) Add a known excess of standard HCl sufficient to dissolve all the carbonate and oxide. Boil-off CO2 cool and titrate the excess acid with standard NaOH using phenolphthalein indicator. c) Subtract the volume of standard HCl used in step (a) from that consumed by the mixture in step (b), the difference is equivalent to CaCO3 in the sample. CaO + 2HC1 = CaCl2 + H2O 2 HCl = CaO Notes: Alcohol is added to prevent formation of lumps of CaO on addition of the sugar solution. B) Determination of Barium Chloride The alkaline earth metal (e.g. Ba2+) is precipitated as its insoluble carbonate by the addition of a known excess of standard Na2CO3. The solution is then boiled, cooled to about 0°C and the excess sodium carbonate is back titrated with standard HCl using phenolphthalein as indicator; multiplying the volume of acid by 2. 19 …………اسرة الفرسان.. pharmaza ………. ………… اسرة الفرسان.. pharmaza …… ………… اسرة الفرسان.. pharmaza The volume used in precipitating the metal ion is obtained by difference. Na2CO3 + BaCl3 = BaCO3 + 2NaCl Notes: 1. Solution must be dilute and heated to 70°C to prevent formation of Ba(HCO3)2. 2. Boiling renders the precipitated BaCO 3 dense. c) Determination of Acetylsalicylic Acid (Aspirin) Esters, such as acetylsalicylic acid, readily dissolve in dilute sodium hydroxide solution and are completely hydrolysed by boiling with an excess of base liberating the sodium salts of acetic acid and salicylic acid. The residual base can then be back titrated with standard acid, using phenolphthalein as indicator. 20