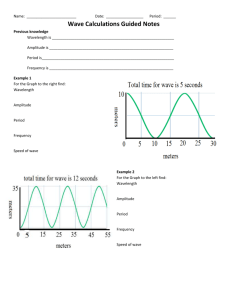
Conceptual Class Notes Semester 2
advertisement

1 Energy Work, Power, and Energy Activity: Burning Calories Walking Activity: Horsepower Forms of Energy Conservation of Energy Lab: Conservation of Energy Machines Efficiency Circular Motion Rotation and Revolution Centripetal Force Temperature, Heat, and Expansion Temperature Heat Specific Heat The High Specific Heat of Water Thermal Expansion Heat Transfer Conduction, Convection, and Radiation Radiation and Newton’s Law of Cooling Activity: Coffee Lab 2 Thermodynamics First Law of Thermodynamics Second Law of Thermodynamics Carnot Heat Engines Vibrations and Waves Wave Description Interference Standing Waves Doppler Effect and Shock waves Sound Properties of Sound Loudness Making Sound Louder Lab: Calculating the Speed of Sound Sound Interference Light Properties of Light Transparent and Opaque Materials Polarization 3 Reflection and Refraction Reflection Refraction Dispersion of Light and Rainbows Total Internal Reflection Color How we see color Light Addition Light Subtraction Color Wheel Lenses Concave and Convex Lenses Ray Diagrams Properties of Lens Images Activity: Calculating Focal Lengths Semester Review 4 Chapter 9 Highlights: Work, Power, and Energy Forms of Energy Conservation of Energy Machines Efficiency Key Terms Energy Power Heat Kinetic Energy Potential Energy Fulcrum Lever Inclined Plane Work Work-Energy Theorem Potential Energy Law of Conservation of Energy Work-Energy Theorem Mechanical Advantage Pulley Efficiency 5 What is energy? Energy is the ability to do work What does it involve? Mass, motion and/or position Does this shotput have energy when it is on the floor? No, it will not do anything. What happens when the shotput is lifted above the floor? It has energy do to it having the ability to do work on other things. What happens when the shotput is kicked? It has energy to do work too. How do we know that each of the two previous situations, the shotput had energy? Explain It will cause damage. It will create a force acting on other objects when it collides. Energy – (units = Joules)- the ability to do work. Work – (units = Joules)- the force applied parallel through a distance that changes the energy state of an object. W = F d Where did the energy to do the work come from? The work done by the person lifting the object – the work came from the chemical energy of the food that was eaten. Do activity: Burning Calories while walking 6 How far do you need to walk in order to burn off the calories in one snack size candy bar? Calories in one candy bar = _____________ Cal. = ____________ J Weight of student = ____________ lbs. = ___________ N the center of gravity of the student is raised and lowered with each step. One step changes the persons center of gravity = __________ cm Therefore the total change in distance is (x 2) = __________ cm Calculate the work performed by the student to do one step: Calculate the number of steps to burn off the equivalent amount of Joules in one candy bar: Calculate the distance traveled to burn off the candy bar: The distance of one step = ___________ m Apply the efficiency of a person to get the actual distance needed to burn off the Calories, use 15%. 7 What is the difference between these two situations? Weight lifted to desk by a student: Weight lifted to desk by teacher: Student lifted it faster – in less time. Do they both involve the same amount of work? Yes, same distance and same weight. Power – (units = Watts)- the rate of work done per unit time, the change in energy per unit time. P = W/t = F d /t = F v American units = horsepower SI units = watts Why aren’t light bulbs measured in hp? Too large of units, hp is related to energy production How many watts does your favorite car produce? How many watts can you produce? How can you figure it out? Time how long it takes to do a certain amount of work. Do activity: Do you have more horsepower than a lawn mower? 8 Running up the stairs: The work performed is the force of gravity on yourself multiplied by the vertical height of the stairs. Measure the height of one step and then multiply by the number of steps on the stairs to get the vertical height. H = ____________ m Total height = __________ m Measure the time you took to perform the work to calculate the power in your body: t = ____________ s Measure your weight = ___________ lbs. = _____________ N Calculate your power: 9 1. How much work is done on a 100 N rock that you carry horizontally across a 10 m room? No work. You’re not changing its energy state. 2. How much work is done on a 100 N rock that you lift in the air a distance of 10 m? W = F d = 100N (10m) = 1000J 3. What would be the power you develop if you lifted the rock from #2 in a time of 10 seconds? P = 1000J / 10s = 100 W 4. If you lifted the previous rock twice as fast, how much power would you need to develop? 200 W, twice as fast means, ½ the time, twice the power Assignment Ch. 9: 1-5, 33-37, 44 10 When the shotput is on the ground how much energy does it have? None. Can’t do work. How do you give it energy? Do some work lifting it or pushing it. How do you know it has gained energy? It can do damage, i.e. work, when it collides. Demo: shotput on cinderblock Work-Energy Theorem – the work done on a system is equal to the change in energy of the system. Work is a vector and has a direction associated with it! When force is in the same direction as the distance covered: Positive work means an increase in energy. Ex. when you press the gas pedal in a car When force is in the opposite direction as the distance covered: Negative work means a decrease in energy, or a release of energy. Ex. a car coming to a stop 1. Kinetic Energy – (KE) – (units = Joules)- energy of motion KE = ½ m v2 2. Potential Energy – (PE) –(units = Joules)- energy due to position, stored energy. (Gravitational) PE = m g h Gravitational potential energy – stored energy due to height When combined in the work-energy equation: W = KE + PE 11 1. In the demonstration of lifting a 5 kg shotput 3 m in the air, it required how much work? W = PE = m g h = 5 (10) 3 = 150 J 2. What is its change in potential energy? 150 J 3. If the shotput was then dropped, how much kinetic energy would it possess right before it strikes the cinderblock? 150 J 4. How fast was it going right before it struck the cinderblock? KE = ½ m v2 = ½ 5 v2 = 150 J : v = 7.75 m/s 5. How much kinetic energy does the shotput have half-way down? ½ KE = 75 J 6. How fast is the shotput going half-way down? Explain why. 75 J = ½ 5 v2 : v = 5.48 m/s 7. What happens to the energy of the shotput when it hits the cinderblock? It is turned into the KE of the cinderblock pieces, but eventually it goes into heat. Assignment Ch. 9: 6,7,38-42 12 What will happen to the student when the pendulum is released? Nothing. Demo: pendulum and student Why does it do this? The pendulum doesn’t have enough energy to get back to its original height. Energy is lost due to friction with the air. Law of Conservation of Energy – energy cannot be created nor destroyed, only transformed from one form into another, where heat is lowest form. The total energy before is equal to the total after. Identify the two kinds of energy as it applies to the energy transformations in the following examples: 1. PE 2. PE + KE 3. KE 4. KE + PE 5. PE Identify the points on the “roller coaster” that have max./ min. kinetic and max./ min. potential energies. How do you know their locations? Demo: match box cars and track Why, in general, are the successive hills on a rollercoaster lower than the previous hill? Energy is lost due to heat and can’t get back to original height 13 Why does the car stop at the end of the “roller coaster?” All of the energy was lost. Where does the initial energy of the coaster go? It turned into heat. Heat – waste energy due to energy conversions and friction. Can we ever get that energy back? No. it is lost forever into space. What determines the max. speed of a rollercoaster? The initial height of the coaster. Can you calculate it out? How? (remember gravity is accelerating the rollercoaster and that mass doesn’t matter) By the conversion of PE into KE at the bottom of the first hill – it is mass independent due to gravity. 1. The vertical drop of a rollercoaster is equal to a distance of 100 m. What is the coaster’s speed at the bottom of the hill? PE = KE, m (10) 100m = ½ m v2 10 (100) = ½ v2 : v = 44.7 m/s ~ 100 mph 14 2. An archer puts a 0.3 kg arrow to a bowstring. An average force of 200 N is exerted to draw the bow string back a distance of 0.7 m. a. How much energy is being stored in the bowstring? W = PE = 200 N (0.7m) = 140 J b. Assuming all of the energy goes into the arrow, with what speed does the arrow leave the bow? PE = KE = 140 J = ½ (0.3) v2 : v = 30.6 m/s c. If the arrow is shot straight up, how high will the arrow rise? PE = PE = 140 J = 0.3 (10) h : h = 47 m Do lab: Conservation of Energy Assignment Ch. 9: 8-11, 13, 51, 54 15 To demonstrate the conservation of energy through projectile motion PE = KE The amount of PE of a matchbox car at the top of the race track is equal to the total amount of KE at the bottom of the race track. By measuring the initial PE of the matchbox car, one can calculate the KE and hence the velocity of the matchbox car, and predict the range of the car using projectile motion equations. h x Using a matchbox track, set-up a ramp on a lab table as shown. A matchbox car will be used as the projectile since its mass is large compared to the number of moving parts - this will reduce rotational inertia and friction - and will give a more accurate prediction. Mark a starting position at the top of the track using your pencil and measure the height of this position relative to the table top. This will be used to calculate the initial PE of your matchbox car and ultimately it will determine the velocity the car will travel off the table top. Be sure to position the track at a height where the matchbox car will not hit the cabinets, or position the track away from the cabinets. 1. Using the conservation of energy equation, calculate the speed with which the matchbox car leaves the table top horizontally. Height of starting position = ____________ m 16 PE = KE 2 m g h = 1/2 m v What drops out of the equation? Does that make sense? Calculated speed = ______________ m/s 2. Roll the matchbox car down the ramp 8 times and create a table of range values and average range. Trial Range 1 2 3 4 5 6 7 8 Average Range = 3. Calculate the average speed of your car by dividing your average range by the time spent in the air = 0.43 seconds. 17 4. Do an error analysis to see how close you were to your predicted speed. % Error = |(actual - theoretical)| theoretical 1. Describe what happens to the kinetic energy of the matchbox car as it rolls down the ramp. 2. Describe what happens to the potential energy of the matchbox car as it rolls down the ramp. 3. How close were you to the actual speed? 4. What were some of the factors that influenced the matchbox car and its speed? 5. Would the matchbox car ever have a speed greater than what you predicted? 6. Given the experiment, do you think that the energy of the matchbox car is being conserved? What other form of energy is involved between the car and the track that would change the car’s energy but not the total energy? 18 Cranes are a compound machine. That is, they are composed of more than one simple machine. What exactly is a machine? Machine – a device that changes the force and/or direction of a force Neglecting friction a machine can be 100% efficient therefore: work input = work output Fin x distancein = Fout x distanceout Work – force acting parallel through a distance that changes the energy of an object Mechanical Advantage (MA) – a ratio that describes how much a force is multiplied. MA = Fout/ Fin 3 Simple Machines: 1. Lever – a machine composed of a rigid bar that will rotate about a fixed axis. *MA = din / dout Lever arm –the distance the input force is to the fulcrum Most efficient machine Fulcrum – the fixed axis of rotation or hinge o 1st class lever – fulcrum is between input and output forces ex. teeter-totter, pliers o 2nd class lever – output force is between the fulcrum and input ex. wheel barrow, nut cracker o 3rd class lever – input force is between the fulcrum and output force ex. boom crane, your arms and legs 19 2. Pulley – rotating lever that uses ropes or cables to change the direction of a force, or with a system of pulleys can multiply forces MA = 5 MA for a pulley is simply found by counting the number of ropes pulling up on the weight! 3. Inclined Plane – (ramp or screw)- a machine that increases input distance to decrease input force. A screw is a ramp wrapped around a shaft. The MA for a ramp can be found by dividing the ramp length by rise or height of the ramp. o For a screw it is called pitch 1. A student pushes down on a lever with a force of 10 N a distance of 1m. How high will an 80 N rock be raised? What is the MA for this machine? 1/8 m, MA = 8 2. Your arm is a 3rd class lever. The MA is about 0.2. If you lift a 30N weight, how much force is your biceps muscle pulling on your forearm? MA = Fout / Fin 0.2 = 30 N / Fin ; 30 N /0.2 = 150 N 20 3. MA for a lever can be described as the ratio of length from the fulcrum. If your input force is 5 m from the fulcrum, and the output force is 1 m from the fulcrum, what would be the MA for this lever? MA = 5 4. What is the MA for a pulley when you apply a force of 150 N to pull up on a 900 N weight? How many ropes are pulling up on the weight? MA = 900 / 150 = 6 5. A force of 40 N is used to push an object up a ramp a length of 2.5 m. The height of the ramp is 1 m. What is the ramp’s MA? What is the object’s weight? MA = 2.5 m / 1 m = 2.5 : MA (Fin) = Fout = 100 N 6. If a block of ice is pushed up a smooth incline a distance of 6 m, to lift it a vertical distance of 1 m. a. What is the ramp’s mechanical advantage? MA = 6 b. If the block weighs 300 N, how much force would be required to push it up the ramp? Fin = 50 N c. How much work was done on the block of ice pushing it up the ramp? W = F d = 50 N (6 m) = 300 J d. How much potential energy will the block have at the top of the ramp? PE = mgh = 300 N (1 m) = 300 J e. Compare the answers to c and d, what do you see? Explain your answer. They are the same, because work input = work output f. If the block of ice were to accidentally slide down the ramp, how much KE would the block have? PE = KE, it would have 300 J of KE 21 g. How fast will the block of ice be traveling at the bottom of the ramp? KE = ½ m v2 : 300 = ½ 30 kg (v2); v = 4.47 m/s 7. Use the diagram at the right to answer the following questions: a. What is the mechanical advantage of the pulley system? MA = 5 b. If the crate weighs 1000 N, how much force will be required to lift the crate? Fin = 200 N c. If you pull down on your end of the rope a distance of 50 cm, how high will the crate be lifted? H = 10 cm d. If you do 5000 J of work to lift the crate, how much potential energy will the crate possess? PE = 5000J e. How high is the crate when you did the 5000 J of work? PE = mgh = 5000J = 1000 N (h); h = 5 m Assignment Ch. 9: 15-18, 47 22 What is the efficiency of your car? Only about 30% efficient, it is also a heat engine – discussed later. What does efficiency mean? Efficiency – is a ratio, expressed as a percent, that relates the amount of actual work output to the actual work input. Where does this energy difference go? Heat due to energy conversions and friction. Is it possible for machines to be 100% efficient? What causes the efficiency of a machine to go down? Machines will always be less than 100% efficient due to friction. If a machine is 100% efficient, then all of the work input = work output. Eff. = AMA / IMA = Actual Mechanical Advantage Ideal Mechanical Advantage = Wout / Win = Ideal input force Actual input force What is the efficiency of a textbook sliding on a ramp? 1. A child on a sled with a total weight of 500 N is pulled up a 10 m slope that elevates her a vertical distance of 1m. a. What is the ideal mechanical advantage of the slope? IMA = 10 b. If the slope is without friction, and she is pulled up the slope at a constant speed, what will be the tension in the rope? Fin = 50 N c. If the slope has friction, and the rope tension was 100 N. What is the actual mechanical advantage? AMA = 5 23 d. What would be the efficiency of the slope that has friction? Eff. = AMA / IMA = 5/10 = 0.5 or 50% e. If a sled with a total weight of 800 N was to be pulled up the slope, what force would be needed if friction were present? AMA = 5; Fin = 160 N 2. A pulley is set up so that 4 ropes are pulling up on a piano. The piano weighs 1000 N. a. What is the ideal mechanical advantage of this pulley system? IMA = 4 b. If it were a frictionless pulley, how much force would be necessary to lift the piano? Fin = 250 N c. If friction is present, and it actually took 300 N of force to lift the piano, what would be the actual mechanical advantage? AMA = 3 1/3 d. What would be the efficiency of the pulley with friction present? Eff. = 3 1/3 / 4 = 0.83 or 83% Assignment Ch. 9: 19-21 24 Write down key terms with their definitions for each chapter. Write down the equations used in each chapter. Solve the following example questions: 1. Work is required to lift a heavy barbell. How many times more work is necessary to lift it 3 times as high? 2. If a barbell weighs 120 N, and it is lifted a distance of 2 m, how much work was required? If it were lifted a distance of 6m? 3. What if lifting the barbell in #2 was done in a time of 3s, how much power was developed in both cases? 4. A 50 kg object has a velocity of 10m/s, what is it’s kinetic energy? 5. If the previous object is traveling twice as fast, how much kinetic energy will it possess? Three times as fast? 6. A 10 kg rock on the edge of a 100 m cliff has how much potential energy? If it were to fall, how much kinetic energy will it have right before it strikes the ground? How fast will it be traveling? 7. In what two ways can a machine alter an input force? 25 8. What is the mechanical advantage of a machine that applies a force of 800N when a force of 80 N is applied to lift a heavy weight? If an input distance of 1m was applied to the machine, how high was the weight lifted? 9. What is the relationship between the MA and the ropes on a pulley? 10. What is the mechanical advantage of a ramp that is 10m long that has a vertical distance of 2m? 11. What would the amount of force required to lift a 500 N object using the ramp in #10? Assume there is not friction. 12. If friction were present in the ramp from the previous example and it required 150 N of force to push it up the ramp, what is the efficiency of the ramp? 13. If a worker uses a pulley to lift a 5000N piano and notes that for every 2 m of rope he pulls down the piano is lifted 0.1m. What is the ideal mechanical advantage of this machine? How many ropes are pulling up on the piano? How much force did the worker apply to lift the piano? If it actually required an input force of 300N, what is the machines’ efficiency? 26 Chapter 10 Highlights: Rotation and Revolution Centripetal Force Key Terms Rotation Linear Speed Centripetal Force Centripetal Acceleration Revolution Rotational Speed Tangential Speed Artificial Gravity 27 How do the planets move around the sun? Did you know that the earth is traveling at a speed of about 67,000 mph? Where is it going? In a circular orbit. Nowhere, it is trapped by gravity in a circle. What is circular motion? Movement around an axis. What are the components of a circle? Radius – distance from the axis to the edge of the circle Diameter – distance across a circle = 2r Axis – center of the circle of rotation Circumference – distance around a circle = 2r To have constant circular motion, what must you have? o Constant _Radius_ o Constant _rotational speed_ There are two kinds of circular motion, what’s the difference? Rotation – movement about an axis inside of itself ex. The earth rotates about its axis. Revolution – movement about an axis outside itself ex. The earth revolves around the sun. A merry-go-round rotates about its axis, while you revolve about its axis. Demo: student on turntable Linear speed – distance covered per unit time Rotational Speed – (angular speed)- how fast something spins about an axis. w = 2 rotations/time 28 Tangential Speed – linear speed but around a circle Demo: rubber stopper on string vT = r x w Where r is the radius of the circle or curve and w is the rotational speed. It can also be found by taking the circumference divided by the time. 1. How fast is this rubber stopper going around my head? 2. How fast is the student rotating on the platform? With arms extended, how fast are the fingers going versus the shoulder? Why? 3. What happens to the rotational speed when the rotating student brings the hand weights in toward the axis? Why does this occur? Rotational speed increases. The speed of the mass wants to stay moving at the same tangential speed, Newtons 1st law, but the radius is now smaller, therefore the number of rotations per unit time increases. 1. Which part of the earth’s surface has the greatest rotational speed? Which part has the greatest tangential speed? They all have the same rotational speed. The equator has the largest radius, and as a result, has the largest tangential speed. 29 2. On the merry-go-round at Six Flags, the horses at the outer edge times farther than the ones toward the center. If you sat on the horses you would experience 0.4 RPM and a tangential velocity of What will be the rotational and tangential velocity of the outer Rotational is 0.4 RPM, and 6 m/s tangential speed are 3 center 2 m/s. horse/ 3. Trains ride on tracks that are equidistant. When a track goes around a curve, which track is actually longer than the other, the inner track, or the outer track? When a train goes around a curve, which part of the train are you actually traveling faster, the inner or the outer part of the train? The outer track, it has to travel farther in the same amount of time. The outer train, moving about in a train going around a curve can sometimes challenge your balance! Assignment Ch. 10:1-7, 29, 31, 33 30 What happens when you swing a penny balanced on hangar? Nothing. It stays on. What happens when you swing water in pan around your head? Nothing. The water stays in. What keeps the rubber stopper in circular motion? The string creates a tension force. What keeps your car in a curve when you turn? Friction. Why do all of these work the way they do? They are all acted on by a centripetal force. Centripetal Force – (FC) force that causes circular motion. It is a force that acts perpendicular to the velocity of an object towards the axis of the circle. FC = m ac Assumed to be constant circular motion o Constant speed in a circle o Constant radius Center of a circle is called the _axis___. This force is directly toward the center of rotation. Centrifugal Force – a “circular” force that pushes objects outward but is based on the object’s inertia. Fictitious force It exists from the observer’s inertia Centripetal Acceleration – (aC) the rate of change of direction per unit time. Recall the definition of acceleration: Depends on two things: o 1. speed o 2. radius Applying this to circular motion: ac = v2 / r 31 NASA is presently working on a space craft that will be able to transport astronauts to Mars. The trip itself will take months. After such a long time in space without gravity, the astronauts’ bones and muscles will be too weak to land safely on Mars. How would NASA be able to simulate gravity so the astronauts could survive? Use a centripetal force What must the centripetal acceleration be equal to? 10 m/s2 to simulate gravity’s acceleration Calculate the tangential speed of a space station if the station has a radius of 100 m. v = 31.6 m/s Videos: space station video clips Assignment Ch. 10: 8-14, 36, 39, 42, 43 32 Chapter 21 Highlights: Measuring Temperature Heat Specific Heat The High Specific Heat of Water Thermal Expansion Key Terms Temperature Celsius Kinetic Energy Absolute Zero Thermal Energy Thermal Expansion Fahrenheit Kelvin Heat Thermal Equilibrium Calorie Specific Heat 33 How do you know if it will be a nice day outside or not? Usually by its temperature. How can you tell if you are sick? Temperature How do you know your food is cooked properly? Temperature Temperature – (everyday definition) – how hot or cold something is. Temperature is scientifically determined by: 1. The type of molecules – certain molecular structures can store more energy than others and can vibrate in more than one direction. 2. Mass of the molecules – more mass at a particular speed results in more energy. 3. Speed of the molecules - faster moving results in a higher temp. Temperature – (scientific) – is the average KE of the molecules Kinetic Energy (KE) –energy of motion, determined by mass and speed. as molecular motion increases: temp. increases atomic MASH pit: the more violent the atomic collisions the higher the temp. and the material will expand. use for thermometers – mercury or alcohol expands at higher temp. the expansion is an indicator of the energy the material possesses 34 Temperature Scales: Celsius – based on the freezing and boiling pt. of water, where 0oC water freezes or melts, 100oC water boils or condenses. Fahrenheit – based on the freezing pt. of salt water (ocean water) 0oF, and human body temp. 98.6oF Kelvin –(SI unit of temperature) – uses the Celsius scale but without negative numbers. Predicts an absolute zero temp. F = 1.8C + 32o K = C + 273 Temperatures of freezing water, and boiling water on the different scales: C F K Freezing water Boiling water 0 32 273 100 212 373 Absolute Zero – the universal lowest temp. predicted by Kelvin in which all molecular motion stops, can never be attained but have come extremely close. -273oC, -460oF, 0K Convert today’s temperature to Celsius and Kelvin. Convert 40oC to Fahrenheit and Kelvin. 104oF, 313 K 35 Which of the following will increase a bucket of room temperature water the most? Why? a. A small beaker of boiling water b. A large beaker of 50oC water Even though the small beaker of water is at a higher temp., the large beaker had more thermal energy. Heat – (units = calories (joules) )- the flow of thermal energy from one object to another. Heat flows from _high temp_ to _low temp_. Thermal Energy – (units = Joules )- total internal KE of the molecules Heat is the result of the release of thermal energy What quantities determine the internal energy of a substance? 1. Temperature – average KE of the molecules in a substance 2. Mass – more mass at a given temp. will have more energy 3. Type of material – some molecules can store more energy than others – related to the specific heat of a substance. Thermal Equilibrium – when the flow of heat stops – occurs when substances are at the same temp. (NOT the same internal energy) How do you measure the heat? Measure the gain in energy of a known substance (water) for comparison to other substances. 36 Listed in Nutritional Information on various products: 1 Cal. = 1000 cal 1 cal = 4.184 J calorie – unit of energy gained by 1 gram of water to increase its temp. by 1oC. Questions: 1. A certain amount of energy is given to a heating plate and it raises 1L of water 2oC. What would the same amount of energy do to the temperature of 2L of water? o 1C 2. Room temperature is about 70oF. What would be room temperature in Celsius and Kelvin? o 21 C, 294 K 3. A thermometer is in a container half-filled with 20oC tap water. What will be the water’s temperature after the following: a. An equal amount of 40oC water is added to this beaker. 30oC b. An equal amount of 0oC water is added to this beaker. 10oC c. An equal amount of 100oC water is added to this beaker. 60oC True or False. A red hot piece of metal is added to a bucket of cool water. a. The decrease in temperature of the metal is equal to the increase in temperature of the water. F b. The quantity of heat added to the water is equal to the quantity of heat lost by the metal. T c. The metal and the water will become the same temperature. T d. The final temperature of the metal and water is halfway between the initial temperatures of each. F Assignment Ch. 21: 1-10, 56 37 What determines the amount of thermal energy contained in an object? 1. Temp. 2. mass 3. Type of material Heat – (units = calories) – (“Q” in the equation) – flow of thermal energy Specific Heat Capacity – (units = cal/g oC) – (“c”in the equation) – relates the amount of energy gained or lost per unit mass of a certain material. Water’s sp. Heat = 1 cal/g oC 𝑸 = 𝒎(𝒄)|𝑻𝒇 − 𝑻𝒊 | Where: Q = heat exchanged in calories m = mass of the substance c = specific heat of the substance Tf = final temp. of the substance Ti = initial or beginning temp. of the substance Examples: 1. Heat was added to 100 mL of water raising its temperature from 20oC to 30oC. How many calories of heat were added to the water? Q = 100g (1) (30-20) = 1000 cal 2. A 200g piece of iron is placed into 300 mL of 20oC water. The water’s temperature increases to 25oC. The spec. ht. of iron is 0.11. What was the initial temperature of the iron? Qiron = Qwater : 200 (0.11) |(25 – Ti)| = 300g (1) (25 – 20) 22 |(25- Ti)| = 300(5) 22|25- Ti| = 1500 |25- Ti| = 68.2o Ti = 93.2oC Assignment Ch. 21: 11-13, 22-28 38 Why does water have such a high specific heat? It has strong molecular bonds. What are the bonds that hold water together? Covalent, and hydrogen bonds Remember, specific heat is comparing energy per unit mass – stronger bonds means more energy required to pull them apart. What are some uses for water because of its high specific heat? Extinguishing fires Heating and cooling homes Cooking Additives can change water’s specific heat: o Anti-freeze – increases the specific heat which makes water boil at higher temps. Does the high specific heat of water affect the climate and weather of cities near large bodies of water? Yes. It makes it more temperate – less temp. extremes. Which would have a higher specific heat: water or land? Water Which one would heat up faster in direct sunlight? Land Which one would cool down slower at night? Water In the summer time, what happens to the wind in Milwaukee at night? During the day? It shifts. Wind blows out to the lake at night – air above water is higher temp. – land has cooled off quicker. Wind blows inland – air above the land is higher temp. due to sunlight heating it up rapidly. In the winter time, what happens when the wind blows across Lake Michigan? It takes the warmer moister air from the lake and creates lake-effect snow when it reaches the colder, freezing air above land. 39 What about the seasons? The peak sunlight, the largest amount of sun energy occurs in late June, yet the highest temperature days don’t occur until August. Why are the seasons delayed approx. 1 ½ months from the peak solstices? It takes some time for the land to cool off enough, or warm up enough to reach peak temps. Why does England have mild temperatures all year long, and then at night get thick fog? It is surrounded by water which stays at roughly the same temp. all year long. 40 What happens when the brass ring is heated and placed over the ball? The ring didn’t fit before and now it does – the heat caused it to expand. Demo: ball and ring Does this work for all substances? For most substances, when heated causes the material to expand. There are a few exceptions. Thermal Expansion – the volume of a substance increases when heated due to the KE of the molecules increasing. The KE of the molecules collide more frequently and violently as to cause the space between the molecules to grow. MASH pit of molecules Expansion rates: o Gases expand the most – lowest sp. Heat o The lower the sp. Heat the higher the expansion rate Applications: Thermometers Expansion joints in bridges and buildings Rubber filled grooves in asphalt roads and concrete sections in sidewalks Sagging telephone lines Shrink fitting, Induction shrink fitting Bimetallic strips Bimetallic Strips – welded brass and iron – thermostat – when heated the different materials expand at different rates. Water is an exception to the expansion rule due to its open crystalline structure: As water cools down to 4oC it _Shrinks_. As water continues to cool to 0oC it _Expands__. As solid water continues to cool below 0oC it __Shrinks slightly__. Why can you fish in the winter? Why don’t the fish die? Warmer water (4oC) sinks due to it being more dense than ice. Assignment Ch. 21: 15-20, 41-45 41 Chapter 22 Highlights: Conduction, Convection, and Radiation Newton’s Law of Cooling Evaporation Key Terms Conductor Heat Radiation Insulator Newton’s Law of Cooling 42 Everyone has probably had a Poptart from the toaster. Specifically, what is going on when you are toasting your Poptart? It is heating up through the 3 methods of heat transfer. Demo: Poptarts Physically how is the toaster heating up your poptart? 1. Conduction – transfer of thermal energy through contact between materials – conductors vs. insulators. 2. Convection – transfer of thermal energy through the movement of a fluid such as air or water. Hot air rises because it is less dense carrying away thermal energy as it rises. 3. Radiation – energy is transmitted by Infrared light – electromagnetic waves which we call heat in common language. (heat lamp) When you touch the Poptart when it is done, it is __HOT_! Why? The thermal energy was transferred by conduction to your hand by the crust. When you let it stand for a few seconds and eat the Poptart, the crust is _cool_. While the filling inside is _hot_____. Why does this occur? The crust was cooled by radiation and convection. It also has a low specific heat, which means it will cool quickly. The filling can only cool by conduction to the crust - which is an insulator, and it has a very high specific heat so it will retain its thermal energy. Conductor – transmits thermal energy easily due to its loosely bound outer electrons. The electrons will allow IR light to transmit through. Heat conduction can also be felt by touch: Grab onto a conductor at room temperature. It feels __cold__. Why? The metal is at a lower temp. then your hand and it will cause the heat to flow more rapidly from your hand to the metal. The flow of heat out of your hand is interpreted as cold. Insulator – doesn’t transmit thermal energy well and will keep the thermal energy localized. Example: glass with torch 43 Place your hand on an insulator type material vs. the conductor at room temperature. It feels _warm____. Why? Even though the paper is at the same temp. as the metal, it will only transfer a small amount of thermal energy per unit time. 1. If you hold one end of a metal bar against a piece of ice, the end in your hand will soon become cold. Does cold flow from the ice to your hand? No. The heat from your hand is going into the ice. 2. Wood is a better insulator than glass. Yet fiberglass is commonly used to insulate wooden buildings. Why? The air pockets trapped by the fiberglass create a better insulation than the wood. 3. You can stick your hand into a hot pizza oven for several seconds without harm, whereas you’d never touch the metal insides for even a second. Why? The transfer of heat from the hot air to your hand is very low due to the low spec. heat of the air and it is an insulator. Metal is a very good conductor of heat and will transfer almost immediately. 4. You can hold your fingers beside the candle flame without harm, but not above the flame. Why? The heat one gets from holding fingers on the side is IR light. Most of the thermal energy is carried away by convection. Assignment Ch. 22: 1-5, 10, 22, 23, 26 44 Which one will cool off faster a hot cup of coffee left on a counter-top or one placed in a freezer? Why? The one in the freezer. There is a larger temp. difference between the freezer and the cup of coffee. Newton’s Law of Cooling – (or warming) – the rate of cooling is proportional to the difference in the temp. of the objects. Heat flow is proportional to ____temperature________. o Increased rate of cooling is due to a ___larger temp. diff._____. o Decreased rate of cooling is due to a ___smaller temp. diff.___. Why does this happen? Energy always flows from high to low. Heat – energy flow from high temp. to low temp. Evaporation – (condensation is opposite) – cooling process which allows the higher than average temp. molecules to escape, thereby carrying away more than their fair share of energy. Water/Alcohol on skin – How does it feel? Why? Cold due to the alcohol extracting heat from your hand to evaporate. What can you conclude about evaporative processes? It is a cooling process. Why does this happen? I takes thermal energy from its surroundings in order to turn into a gas. Demo: molecules in a jar Graph of molecular temperatures: # of molecules 0 Temp. (K) 45 Does Color have an effect on the rate of cooling and absorption of energy? Yes. Black absorbs/radiates more heat than white or silver. Demo: lamp on colored cans Questions: 1. If a good absorber of radiant energy were a poor emitter, how would its temperature compare with its surroundings? It would always feel cold – constantly absorbing energy. 2. Is it more efficient to paint a heating radiator black or silver? Black, it gives off more energy. 3. Why is it a good idea to keep hot beverages covered? It stops the cooling by evaporation. 4. Why do thermos bottles have a silver coated, vacuumed interior? The silver reflects the IR light back in and the vacuum is a perfect insulator. No molecules to conduct the heat. Do Coffee Lab Assignment Ch. 22: 12-17, 33 46 Chapter 22, problem #34; Suppose that a person at a restaurant is served coffee before he or she is ready to drink it. In order that the coffee be hottest when the person is ready for it, should cream and sugar be added to it right away or just before it is drunk? Predict what you think is the answer to this problem using the effects of temperature difference, color, specific heat, and evaporation on the rate of cooling: Direct measurement of the problem: Pour an amount of coffee into two Styrofoam cups up to the line indentation toward the top of the cups. Immediately add two sugar packets and one creamer to one of the coffees, stir, and measure the initial temperatures of both coffees. With a stopwatch, time 8 minutes, and record the temperatures of both coffees at one minute intervals. At the end of 5 minutes, add the cream and sugar to the other coffee, and record the temperatures. Time 0 1 2 3 4 5 Coffee w/cream and sugar Coffee w/o cream and sugar – add cream and sugar to coffee 6 7 8 47 Does color influence the rate of cooling more in the coffee? Pour an amount of coffee into a Styrofoam cup up to the line indentation toward the top of the cup. Pour an equal amount of hot water at the same temperature in another cup. Measure the initial temperatures of both cups. With a stopwatch, time 8 minutes, and record the temperatures of both cups at one minute intervals. Time Coffee Hot water 0 1 2 3 4 5 6 7 8 Does the sugar influence the rate of cooling more? Pour an amount of hot water into two Styrofoam cups up to the line indentation toward the top of the cups. Add two packets of sugar to one of the cups of hot water. Measure the initial temperatures of both cups. With a stopwatch, time 8 minutes, and record the temperatures of both cups at one minute intervals. At the end of 5 minutes, add two packets of sugar to the other cup, and record the temperatures. Time 0 1 2 3 4 5 6 7 8 Hot water w/sugar Hot water – add sugar 48 Does the creamer influence the rate of cooling more? Pour an amount of hot water into two Styrofoam cups up to the line indentation toward the top of the cups. Add initial temperatures temperatures of both creamer to the other Time 0 1 2 3 4 5 6 7 8 a container of creamer to one of the cups of hot water. Measure the of both cups. With a stopwatch, time 8 minutes, and record the cups at one minute intervals. At the end of 5 minutes, add the cup, and record the temperatures. Hot water w/creamer Hot water – add creamer Does the initial difference in temperature influence the rate of cooling more? Pour an amount of hot water into two Styrofoam cups up to the line indentation toward the top of the cups. Add a small amount of room temperature water, 10 ml, to one of the cups of hot water. Measure the initial temperatures of both cups. With a stopwatch, time 8 minutes, and record the temperatures of both cups at one minute intervals. At the end of 5 minutes, add 10mL of room temperature water to the other cup, and record the temperatures. Time Hot water w/10 mL water Hot water 0 1 2 3 4 5 – add water 6 7 8 Conclusions of the investigations into the rate of cooling of coffee: Explain why the coffee cooled more or less when the cream and sugar were added after 5 minutes rather than right away. 49 Chapter 24 Highlights: First Law of Thermodynamics Second Law of Thermodynamics Carnot Heat Engines Key Terms Thermodynamics Adiabatic Heat Heat Engine Entropy First Law of Thermodynamics Second Law of Thermodynamics Pressure Work 50 How does a Diesel engine work? The heat of compression ignites the fuel air mixture. What happens to the temperature of air when you compress it? It heats up. Demo: bike pump, air compressor Why does this occur? The molecules are forced into a smaller volume causing more violent collisions with the container and each other. Demo: bouncing ping-pong ball Where does this energy come from? Came from the work done compressing the gas. Would it be equal to the amount of energy we put into compressing it? Yes. But there is some from the friction of the pump. Does this process work in reverse? What happens when we expand a gas? Yes. The sudden expansion causes the temp. to go down. Less violent and less frequent collisions with an increased space between the molecules causes an overall lower temp. Demo: compressed air on hands Thermodynamics – study of the flow of heat. First Law of Thermodynamics – (law of conservation of energy) – the heat added to a system is equal to the energy increase of the system, including any work done by the system. Compression __increases_ the temp. of a gas. Expansion ___decreases___ the temp. of a gas. By ideal gas law: PV = nRT and increase in temp. for a given volume will increase the pressure. If the container has a moveable piston or something similar, the gas will be allowed to expand and do work. Work – (J)- a force acting through a distance that changes the energy of a system. Heat Engines – a machine that converts heat into mechanical energy 51 Heat added = Heat output + Work done Adiabatic Process – an isolated system that does not allow heat to be removed or added that changes the pressure and volume of the system. PV = energy of the system. Explanation of an adiabatic heat engine process: 2 1 Temp./Pressure 3 4 Volume 1. An explosion superheats the gases inside an engine, creating a sudden increase in temp./pressure. 2. The increase in pressure moves a piston. As the piston moves, the volume increases, and the pressure and temperature decrease. The exhaust is released. 3. The piston allows in air and gas by decreasing pressure and temperature. 4. The piston does work compressing the air and gas to a higher pressure and temperature. The area under the Pressure/Volume curve is the available energy to do work – the larger the area = more work. This process works in reverse = Refrigeration cycle. If you do work decreasing the pressure of a gas by expanding it, it will decrease its temperature. external work done = decrease in internal energy This does not violate the conservation of energy due to more work being done than a decrease in internal energy. How does this process apply to weather and climatic conditions? Differences in temp. drive weather patterns and wind due to the flow of heat. What happens to the atmospheric pressure as you increase height in the atmosphere? It decreases. 52 If you had a balloon filled with helium and let it rise in the air, what would happen to the size of the balloon? It would expand and get bigger. What happens when you expand a gas? It cools. Apply this principle to rising air currents with water vapor: Assignment Ch. 24: 5-12, 33-36 53 How many different kinds of engines are there? Dozens. What basic principles do they use? Compress an air fuel mixture. Ignite it. Control the expansion to do work. Demo: engine animations Second Law of Thermodynamics –Heat always flows from High temp. to low temp., energy goes from a higher state to a lower state. The amount of work from a high temp. source to a low temp. source can never be 100% Heat Engines – a machine that converts the flow of thermal energy into mechanical energy Demo: steam engine High temp. source = __steam = 100oC = 373 K_ Low temp. source = ___room temp. air = 20oC = 293 K__ What happened to the energy of the fuel source? Where did it end up? Can we ever get the energy back? It was burned and turned water into steam. Some of the energy burned went into steam, but also into the exhaust. But ultimately the energy went into the work done and the heating up of the classroom. We can’t get it back because the heat is radiated away into the surroundings. What happens to the temp. of the room? Temp. went up. Entropy – a measure of disorder of a system, related to the amount of available energy and it is always decreasing This will not happen due to the room (low temp. source) being very large compared to the steam exhaust - as the engine is run more and more until the room temperature gets very hot, will you get as much power from the steam engine? No. the temp. difference comes closer together until there is no flow of heat 54 Carnot Heat Engine – a theoretical frictionless engine that can convert as much thermal energy into work. It serves as an absolute upper limit to heat engines. Max. Eff. = Thot-Tcold Thot What would be the maximum efficiency for the steam engine? Eff. = (373-293)/ 373 = .214 = 21.4% There will always be heat energy lost due to heat to the cold source, there can never be an engine that is 100% efficient. If a heat engine were 100% efficient then all of the fuel that is burned will be converted into work. If all of the energy is converted into work, there will not be a need for a cold source. If there is not a cold source, then what causes the heat to flow? If there is no cause for the heat to flow, then the burning fuel cannot be converted into work! 1. What would be the max. eff for a car engine? High temp = 700K, low temp. exhaust = 300 K. Eff. = (700-300)/ 700 = 0.571 = 57.1% 2. What reduces the efficiency of a heat engine? Friction. Moving parts. Temp. of fuel burn. 3. How could you increase the efficiency of a heat engine? Reduce friction. Reduce the number of moving parts. Increase the source temp. Video: ceramic engines, gas turbine engine 4. Would it be a good idea to cool your room by leaving the door to your refrigerator open? Explain. No. The temp. inside the fridge is low but the back of the fridge gets very hot! 5. If 10 J of energy is added to a system that does no external work, how much will the internal energy of that system be changed? 10 J 6. If 10 J of energy is added to a system that does 4 J of external work, how much will the internal energy of that system be raised? 6 J 55 7. What is the max. eff. of an engine if the hot source and the cold source are the same temperature = 400 K? Eff. = (400 – 400)/400 = 0/400 = 0% It won’t work. 8. What is the max. eff. of an engine having a hot source at 400 K and a cold source at absolute zero = 0 K? Eff. = (400 – 0)/400 = 400/400 = 100% Absolute zero can never be attained by definition. 9. What is the max. eff. of an engine having a hot source at 800 K and a cold source at 310 K? Eff. = (800-310)/800 = 0.6125 = 61.25% This is the absolute limit of efficiency for the internal combustion engine. The push towards electric motors is now stronger due to recent technological advances which make them up to 96% efficient. Electric motors are not heat engines and are not constrained by the Carnot Engine Efficiency model. Assignment Ch. 24: 13-18, 26-29, 38-40, 51-58 56 Write down key terms with their definitions for each chapter. Write down the equations used in each chapter. Solve the following example questions: 1. What is used to measure temperature? 2. Convert 74 degrees F, into Celsius, and Kelvin. 3. Why is it incorrect to say that matter contains heat? What does it contain? 4. Do substances that normally heat up quickly have a high or low specific heat? 5. Compare the specific heat of water to other known substances, how does it compare? Why? 6. Why does a bimetallic strip bend when heated or cooled? 7. Which of these expand the most when heated: Solids, Liquids, Gases? Why? 8. At what temperature is the density of water the greatest? Why? 9. Calculate the number of heat calories when 400g of water is heated from 20 degrees Celsius to 80 degrees. 10. Calculate the number of heat calories lost by a 1200 g chunk of copper metal, c = 0.2, as it cools from 100 degrees to 30 degrees. 57 11. Calculate the specific heat of 600g of an unknown substance when it is cooled in 500ml of water. The initial temperature of the substance is 100 degrees, and the initial temperature of the water is 23 degrees. They both reach thermal equilibrium at 28 degrees. 12. 13. What is the role of loose electrons in heat conductors? What is radiant energy? 14. What color is a good absorber of heat energy? What is a good emitter? 15. What will cool off faster, a cup of coffee that has the cream in sugar added right away, or one in which you wait and then add the cream and sugar? Why does this work? 16. 17. 18. What reduces the efficiency of a heat engine? If an engine is frictionless, is it 100% efficient? Explain the energy cycle for all heat engines. 19. Calculate the max. efficiency of an engine that operates between a high temp. of 900 K and a low temp. of 300 K. 58 Chapter 25 Highlights: Wave Description Interference Standing Waves Doppler Effect and Shock Waves Key Terms Periodic Motion Continuous Waves Transverse Wave Electromagnetic Waves Rarefaction Period Wavespeed Constructive Standing Wave Anti-Node Shock Wave Wave Pulse Mechanical Wave Longitudinal Wave Compression Wavelength Frequency Interference Destructive Node Doppler Effect 59 What is a wave? a periodic disturbance in a medium What does it do? Transfers energy List some examples of waves: Water, sound, light, seismic, electricity, wind, etc. What is the source of all waves? A vibration Periodic Motion - (Pendulum) – a vibration that occurs at regular intervals of time. One period is the time to make one complete vibration Demo: super slinky How do we represent waves? A curvy line Demo: marker on white board - mathematically this is called a: sine curve Wave pulse – a single disturbance in a medium Phase - (conceptually represented) – the direction of the wave pulse Phase up – a pulse above the neutral line Phase down – a pulse below the neutral line Mechanical waves - (2 types) – waves that exist in a matter medium. Ex. water, sound, seismic matter doesn’t move with the wave - only the energy is transferred! 60 Transverse – wave disturbance is perpendicular to the wave speed. ex. water surface waves visual representation: Longitudinal – wave disturbance is parallel to the wave speed. Ex. sound visual representation: Electromagnetic waves – waves created by alternating electric fields, Ex. light What do waves transmit? energy How fast do waves travel? it depends on the type of wave and the medium Each kind of wave possesses the following: amplitude, crest, trough, neutral line, wavelength Crest Amp. Neutral Line Trough Wavelength Representing longitudinal waves: (Sound) Compressions – the crest for a longitudinal wave, it is the area of higher pressure. 61 Rarefactions - (Expansions) – the trough of a longitudinal wave, it is the area of lower pressure. Amplitude – the distance between the neutral line and the peak of the phase, it is related to how much energy the wave carries. Wavelength - (symbol = ) – the distance covered by a wave in one period, the distance of one full crest and trough Period - (symbol = T) the time for one complete vibration or wave. Frequency - (symbol = f) – how many waves or vibrations occur per unit time Looking at the units, what do you think the relationship is between period and frequency? f = 1/T Wavespeed - (symbol = v) – how fast a wave travels through a medium. It is the distance covered per unit time through the medium. You’re at a Railroad crossing and notice that 2 train cars pass by the lights in 1 sec. Each train car is 18 m long. How fast is the train going? V = 2 train cars/ sec. x 18 m / train car = 36 m/s Do a unit analysis to find out what the relationship between wavespeed, period, frequency, and wavelength. Wavespeed equation: v = f 62 What determines the speed of the wave? the properties of the medium. What happens when the frequency of the vibration is increased? the wavelength decreases What happens when the wavelength is increased? The frequency decreases 1. Calculate the wavelength of your favorite tone from the signal generator: The speed of sound in air is 343 m/s. 2. Calculate the period of vibration for the speaker producing your favorite tone from above: 3. Calculate the wavelength of your favorite FM and AM radio station: The 8 speed of light is 3 x 10 m/s. 1. What is the period of a 100 Hz wave? T = 1/100 = 0.01 s 2. A skyscraper sways back and forth with a period of about 4 s. What is the frequency of vibration? f = ¼ = 0.25 Hz Assignment Ch. 25: 1-11, 26, 27, 31, 32, 35, 37 63 What are tidal waves and tsunamis? Large waves that occur in the ocean due to the moon or earthquakes. What are Rogue waves (Freak waves) and why are they different than the other waves? Large waves that will suddenly appear at random. How are these Rogue waves created? Due to constructive wave interference. Demo: superslinky Interference – (2 kinds) – two or more waves that meet in a medium, they will algebraically add together to form a new wave. Constructive – waves meet in phase and create a larger wave. + = Destructive – waves meet out of phase and create a smaller wave. Partial: Complete: + + = = Ex. noise canceling headphones, military helicopters, laser beams Watch video series: Freak Waves 64 What happens when the incident wave from a source interferes with its own reflected wave? A standing wave is formed Demo:superslinky, standing wave animation Standing wave – a stationary wave form created by interference from its own reflected wave Anti-Node – area of max. displacement due to constructive interference Node – area of min. or no displacement due to destructive interference Video: Tacoma Narrows Bridge What is the relationship between the number of AN’s and the frequency of the wave produced? Direct. Increase the freq. you increase the # of AN’s What happens to the wavelength of the slinky when the frequency is increased? Wavelength goes down. What’s happening to the size of the AN’s? AN size decreases 1/2 standing wave: 1 standing wave: 1 1/2 standing wave: 1. Is it possible for one wave to cancel out another wave so that the combined wave amplitude is zero? Yes. Application: noise canceling headphones 2. Suppose you set up a standing wave with only 1 AN. If you shake the slinky with twice the frequency, how many AN’s will appear? If you shook it 3 times as fast? 2, 3 Assignment Ch. 25: 12-14, 22 65 What happens to sound if the source or observer is moving? The pitch appears to change. Demo: Mr. Doppler, Videos Doppler Effect – the apparent shift in frequency due to the relative motion of the source and observer toward – higher pitch away – low pitch works for all types of waves! - transverse, longitudinal, and electromagnetic wave speed 1 2 Boats on water, radar/Laser guns Boat 1 travels against the velocity of the wave crests. Boat 1 will encounter _more_ wave crests per unit time. Boat 2 travels with the velocity of the wave crests. Boat 2 will encounter __less__ wave crests per unit time. Each boat will perceive a different frequency than the frequency measured by a stationary observer. What happens when the boat or any other source travels faster than the wave speed of the material? Wave crests will bunch up and overlap constructively Bow Waves – “v” shaped wave form created by boats traveling faster than the wavespeed of surface waves Shock Waves – cone shaped high pressure area created by an object traveling faster than the speed of sound Sonic Boom – loud thunder clap created by the high pressure of a shock wave Video clips: aircraft breaking the sound barrier Assignment Ch. 25: 15-21, 45-49 66 Write down key terms with their definitions for each chapter. Write down the equations used in each chapter. Solve the following example questions: 1. How is a sine curve related to a wave? 2. Draw a sine curve and label the following: Amplitude, crest, trough, wavelength, neutral line 3. Does the medium travel with the wave? Explain. 4. As the frequency of sound increases what happens to the wavelength? 5. Describe the two different kinds of interference and what creates them. 6. What causes a standing wave? 7. Draw a 1 wavelength standing wave and label the following parts: antinode, node. 8. The Sears tower in Chicago sways back and forth with a period of 8 seconds. What is the frequency of vibration? 9. Calculate the speed of a wave if the crests are 0.3 m apart and two crests pass by a stationary point in 2 seconds. 67 10. Calculate the wavelength of a 200 Hz sound in air, assume the speed of sound is 340 m/s. 11. How far, in terms of wavelength, does a wave travel in one period? 12. What do the concentric circles on the surface of a pond indicate about the wave speed? 13. Does the Doppler effect occur for some types of waves or all types of waves? 14. What effect does the speed of a moving source have on the wave speed of a sound wave? 15. The frequency of a favorite radio station is 103.1 MHz. What would be the wavelength of this radio signal? The speed of light is 3 x 108 m/s.( M = 1,000,000) 68 Chapter 26 Highlights: Properties of Sound Loudness Making Sound Louder Sound Interference Key Terms Sound Trough Infrasonic Amplitude Resonance Standing Wave Beats Crest Pitch Ultrasonic Decibels Forced Vibration Harmonics 69 What is sound? A longitudinal mechanical wave Demo: Paper towel in doorway How is sound created? Any kind of vibration Demo: desk slide, speaker w/frequency generator Does sound need matter? Yes. It is a mechanical wave – no air, no sound Demo: bell in vacuum chamber For a Sound Wave: Crest – area of higher pressure Trough – area of lower pressure What does the speed of sound through the material depend on? The physical properties of the material Different materials have a different speed of sound. Air at room temperature and 1 atm. of pressure, has a speed of 340 m/s. In general, the speed increases 0.6 m/s for every 1oC increase. Increase in temp. – increases molecular collisions – air becomes more elastic at higher temps. Pitch – sound frequency Hearing range for average humans (varies by age and gender) 20 Hz – 20,000 Hz Demo: sound generator Infrasonic – sound frequencies below 20 Hz Ultrasonic –sound frequencies above 20,000 Hz 70 Why do Dolphins or other aquatic mammals, and bats use high frequency sounds? High frequency sounds have very small wavelengths. How does that work? The size of the wavelength will determine the smallest sized object it will reflect off of. As you increase the frequency, what happens to the wavelength? It gets smaller Speed of sound in water ~ 1500 m/s (NOT surface waves! It depends on the composition of the water – fresh water ~ 1300 m/s, and it also depends on temp. and depth. o The atoms are very close together >> dense than air Speed of sound in metals ~ 5000 m/s or more depending on the type of metal. o The atoms are bonded together and very elastic (spring materials) Demo: metal spring phone Back in the 1800’s and early 1900’s people would put their ears to railroad tracks. Why would they do that? Sound conducts better through the metal rails than through air to hear a train coming. Examples: 1. Calculate the wavelengths of sound waves for human hearing: 17.2 m - 0.0172 m 2. How far away is a storm if you see a flash and hear the thunder 3 seconds later? D = s t = 340m/s (3 s) = 1020 m; ~ 1 km Class Activity: Calculating the speed of sound in air Assignment Ch. 26: 1-9, 24-30, 44-48 71 How do we measure the amount of sound coming from a source? Measure the amount of pressure between the crest and trough. Amplitude – for a sound wave – the pressure difference between the crest and the normal medium Decibels – (dB) – unit of measure for the amplitude of sound, based on human hearing and is plotted on a logarithmic scale of base 10. Relative sound intensity increases 10x for every 10 dB increase. exponential scale: 20 dB is __10x_ more intense than 10 dB, and 30 dB is _100x__ more intense than a 10 dB sound. – NOT 20x more intense! Overhead: Decibel scale Demo: Sound meter 1. How many times more intense is a rock concert (130 dB) to a physics class? Physics class = ________ dB 2. How many times more intense is a physics class to a whisper? 3. How many times more intense is a physics class to a jet? Assignment Ch. 26: 34, 35 72 How do musical instruments work? They amplify sound using sound resonance. What is first needed to produce sound? A vibration. Woodwinds – reed, Brass - lips How would you make sound louder? Influence more air to vibrate. Forced Vibration – vibrating a larger surface area to amplify sound. A sounding board is used in pianos, a stretched piece of skin in a banjo, etc. special form of forced vibration Demo: tuning forks and resonance boxes Resonance – when a given column of air vibrates with the same natural frequency of a forced vibration they will reinforce each other and create a sound standing wave. air column resonance – an enclosed space of air vibrates with a natural frequency Demos: PVC pipe resonances with tuning forks and wave generator, tuned intakes, tuned exhaust Standing Waves – created by interfere from its own reflected wave. Harmonics – higher frequencies that are multiples of the lowest frequency that resonate in the same column of air. Do Lab: Finding the Speed of Sound in Air Assignment Ch. 26: 11-17, 36, 39, 41 73 To calculate the speed of sound in air by applying a property of closed tube resonance. The fundamental wavelength of a closed tube is equal to four times the length of the air column. = 4 L By knowing the frequency for the fundamental of the closed tube, and measuring the length of the air column, one can calculate the value of the speed of sound in air. v = f = f (4 L) There is a correction to the air column length, due to the width of the air column, which must be added to the measured air column length for a proper calculation. The correction factor is 40% of the diameter of the air column or: L = h + 0.016m You will be using a PVC tube ~ 0.45 m in length, and a 1 L graduated cylinder filled with water to simulate a variable closed tube. The length of the air column in the PVC pipe is varied by pulling the pipe in and out of the water in the graduated cylinder. 1. Select one of the tuning forks from the card board box and record its frequency in the table. This will be the fundamental frequency for your air column. 74 2. Strike the tuning on the rubber hammer provided and hold the tuning fork above the top of the PVC pipe. Pull the PVC pipe up or down in the water until the loudest tone is made. At the point of loudest tone, resonance occurs, measure the distance from the top of the PVC pipe to the top of the water level in the graduated cylinder. This is the air column length h. 3. Select the other different tuning fork from the box and another one of your choosing from the stand, and do the same procedure, recording the measured values in the table. Tuning Fork Frequency Length of air column = l Corrected Air column Length = L Calculated fundamental wavelength = 4 L Calculated Speed of Sound in Air = v 288 Hz 512 Hz 75 o 1. The accepted value for the speed of sound in air is 331 m/s at 0 C and 1 o atm of pressure. The speed of sound in air is increased 0.6 m/s per 1 C in dry air. Use the air temperature from the classroom to calculate the actual speed of sound in air. actual speed of sound in air = _____________ m/s 2. Do a percent error analysis for each tuning fork to see how close you were to the theoretical accepted value for the speed of sound in air. % Error = |(actual - calculated)| actual 3. How does your calculated experimental value for the speed of sound in air compare to the theoretical value? 4. What were some of the errors involved in the lab experiment? Any assumptions? 5. What other experiment could you do to find the speed of sound in air? 76 How do piano tuners tune a piano? They listen for sound beats. they use a property of sound interference What is interference? When two or more waves meet in a medium. What will happen when these two sound waves meet? They interfere constructively and destructively at certain points. Area of Decreased Pressure (Destructive) Area of Increased Pressure (Constructive) Demo: Combs on overhead, beat freq. animation Beats – are created by interference from two similar frequencies. The closer the two frequencies are, the smaller the beat freq. beat frequency = |(f1 - f2)| Demo: wave generators, resonance boxes, tuning forks 77 1. What would be the beat frequency of a 520 Hz tuning fork sounding at the same time as a 512 Hz tuning fork? 8 Hz 2. If you heard a 5 Hz beat frequency, and you had the 520 Hz tuning fork, what would be the possible frequencies of the other tuning fork? 525 Hz, 515 Hz 3. What is the beat frequency when a 262 Hz and 266 Hz tuning fork are sounded together? 4 Hz 4. What about a 262 Hz and 272 Hz? 10 Hz Assignment Ch. 26: 18-21, 23, 37, 51-53 78 Write down key terms with their definitions for each chapter. Write down the equations used in each chapter. Solve the following example questions: 1. What is the source of all sounds? 2. What is the speed of sound in dry air at room temperature? 3. Compare the speed of sound in air to the speed of sound in water and metals. Explain their difference. 4. How does pitch relate to frequency? 5. What is the average frequency range of human hearing? 6. Calculate the wavelength range of human hearing. 7. In terms of crests and troughs, what are these corresponding parts of a sound wave? 8. What kind of wave is a sound wave? 9. How does temperature affect the speed of sound in air? 10. What effect does using a sounding board on a musical instrument have on the sound it produces? Explain why this works. 11. What happens when a musical instrument produces resonance? 12. How is interference related to beats? 79 13. When watching a baseball game, why do you hear the sound of the bat hitting the ball, after you see it? 14. What two physics mistakes occur in sci-fi movies when an explosion occurs in outer space? 15. How is the amount of sound from a source measured? 16. A 10 dB sound is made. Comparatively, how much more intense is a 20 dB sound? 30 dB? 40 dB? 17. Suppose you have a 512 Hz tuning and you hear a 12 Hz beat frequency when sounded with another unknown tuning fork. What are the two possible frequencies of the unknown tuning fork? 80 Chapter 27 Highlights: Properties of Light Transparent and Opaque Materials Polarization Key Terms Roemer Michelson Transparent Umbra Penumbra Electromagnetic Spectrum Light-year Opaque Light Ray Model Polarization 81 What is light? An oscillating electric and magnetic field – a transverse wave that carries energy, it does not need matter to exist – it travels through space. What did Einstein propose in 1905? Light is tiny bundles of vibrating energy called photons– like water molecules in water waves. What creates light? Vibrating or accelerating charges. Are there different kinds of light? Yes, many different kinds, we only see a small sliver of the entire spectrum. Electromagnetic Spectrum – a chart of increasing frequencies of light based on the light properties and how it interacts with matter. transverse wave Radio Microwave Infrared Visible Ultraviolet X-ray 4 9 12 14 15 17 Gamma 20 Cosmic 24 0----10 ------10 -----10 --------10 -----10 --------10 -----10 -----10 ----> What are the uses for light? Demo: heat lamp, UV lamp How can you measure the speed of light? Roemer (Olaus)– first scientist to prove that light has a speed. ~ 2.24 x 108 m/s Earth-Dec. Earth-June Sun Jupiter Io 82 Michelson – won the Nobel Prize for measuring the speed of light accurately Sodium Lamp Octagonal Mirror Calif. Mt. Valley Scope If the mirror was rotating too slow, or too fast, the light beam wouldn’t be lined up properly and the beam wouldn’t be reflected down the scope. The alignment must be timed just right to get the light to reflect off both mirrors back down the scope, in the same time it took the light to travel the 70 km would then be 1/8 of a turn. o Published speed = 299,799,600 m/s The agreed upon speed of light is 299,792,458 m/s by international standards. The measure of the speed of light is done by atomic clocks. The time it takes light to travel exactly 1 meter = 1/ 299,792,458 seconds. 8 Usually the speed of light, c = 3 x 10 m/s Light-year – the distance traveled by light in one year given the constancy of the speed of light. 365.25 days/year x 24 h/day x 3600s/h = 31,557,600 s (3 x 108 m/s) = 9.47 x 1015 m, 5.88 x 1012 miles 1. Is it correct to say that a radio wave is a low-frequency light wave? Yes. It is an EM wave. 2. Is a radio wave also a sound wave? No. It is a different kind of wave Assignment Ch. 27: 1-6, 24-26, 33-34 83 List some examples of transparent materials: Glass, plastic, water, alcohol, gems, air List some examples of opaque materials: Wood, concrete, metals, etc. What makes a material transparent?(Scientific explanation) If the material does not resonate at the frequency of visible light. Transparent – allows light through if the material does not resonate, or vibrate at the same natural frequency, it passes from atom to atom through the material. Opaque – blocks or absorbs the transmission of light due to the material resonating or vibrating at the same frequency and the light gets absorbed. Light Ray Model – (light ray approximation) –light travels in straight lines in all directions from a source – a particle property of light. Demo: Pinhole camera Assignment Ch. 27: 10-17, 39 84 What happens when one of the polarizers is turned 90 degrees relative to the other polarizer? It turns black. It absorbs the light. What happens when you view the light reflections from the desk tops through the polarizer? Rotate the polarizer. The reflection disappears. What happens when you view an LCD screen through a polarizer? It turns black as you rotate the polarizer. What can you conclude about an LCD screen? It uses polarizers to produce the black pixels on the screen. What happens when you view the sky on a sunny day through a polarizer? The blue sky appears to darken as you rotate the polarizer. Why do all of these occur? All of these use or have polarized light. Polarization – the vibration of light in a single direction – similar to shaking a rope or spring, it only vibrates in the direction of vibration. Light is a transverse wave - this is a property of only transverse waves. Light emitted from bulb is radomly polarized Only vertically polarized light is allowed to pass through Polarizer resonates in only one direction all other light is absorbed If light and object are not in resonance – it passes through If light and object are in resonance – it gets absorbed Polarizers aligned perpendicularly will – absorb the light A perfect polarizer will only absorb ___ ½ __ the light from a randomly polarized source. Assignment Ch. 27: 20-23, 46-48 85 Chapter 29 Highlights: Reflection Refraction Dispersion of Light and Rainbows Total Internal Reflection Key Terms Law of Reflection Diffuse Reflection Snell’s Law Critical Angle Regular Reflection Refraction Dispersion Total Internal Reflection 86 How do mirrors work? They will absorb and reradiate light due to the free outer electrons What happens when you view at a window from inside your house at night? Why does it do this? You see your reflection, some of the light is reradiated back to you, most of the light is passed through. What is the light ray model for light? Light travels in straight lines What happens when light encounters matter? 1. absorbed – light is turned into heat 2. transmitted – light passes through from atom to atom 3. reflected – light is reradiated off the surface due to free outer electrons in the atoms Demos: laser on mirror, light ray box on mirror Law of Reflection – light will reflect at equal angles when measured relative to the normal, like a bouncing ball. Identify the angle of incidence, angle of reflection and the normal in the diagram: Normal – imaginary perpendicular line From the surface at the point where the light ray strikes the plane parallel light rays reflect in a specific way Two kinds of reflections: Regular – reflects at equal angles, like a mirror or other metallic surface Diffuse – reflects at many different angles and spreads the light out, all other reflections – it’s how we see each other. Assignment Ch. 29: 2, 4-6, 11, 31, 32, 34 87 What happens when a pencil is placed in a beaker of water? It appears to be larger and bent Why do you see fish underwater at a different location than where it actually is at? The light rays are being bent away from their normal direction Refraction – the bending of light rays due to the difference in the speeds of light of two different transparent materials. Light is usually slower in more dense materials. The incident light is parallel to the light ray transmitted on the other side when the object’s sides are parallel The amount a light ray is refracted depends on the difference in densities of the two materials. o Less dense to more dense – the light is bent toward the normal. o More dense to less dense – the light is bent away from the normal. What happens to the light rays as the angle of incidence is increased from 0 to 90 degrees? It bends more and more towards the normal. Demo: light ray box on glass Diagram of a light ray through a more dense material: Air Glass Air 88 Snell’s Law – describes how much the speed of light will change, and therefore bent, due to the density of the transparent material. The index of refraction describes the optical density of a transparent material. What is the speed of light? In a vacuum = 3 x 108 m/s What happens to the speed of light as it enters a new material? If it is more dense, it slows down, less dense it speeds up but never greater than in a vacuum Why does it do this? Light has to travel from atom to atom through the substance, which, essentially, slows down the speed of light. If this occurs, then as light enters a new material the frequency of the light must remain the same (red light is still red light in glass). f air f glass v = c/n, where c = speed of light in a vacuum, v is the speed of light in the transparent material, and n is the index of refraction of the material Assignment Ch. 29: 12, 13, 15-21 89 Why do we see rainbows? White light is actually a combination of all the colors Demo: rainbow pictures What happens when white light travels through a prism? White light is refracted into the different colors Why does it do that? Each light frequency has a slightly different speed through the material Demo: light ray box and prism Dispersion – the refraction of white light into the different colors due the speed of the light depending on the frequency. Related to the index of refraction – Higher speeds means less refraction Each light frequency experiences a slightly different index of refraction – red light is smallest, violet light has the largest. Each light frequency will travel at slightly different velocities through material. Application to Snell’s Law: Rainbows Water Droplet White light Violet Red Assignment Ch. 29: 22-25 90 How does fiber optics work? Has different kinds of glass that create total internal reflection What happens to the light beam as the glass is rotated 90 degrees? The light ray refracts and bends more and more until it doesn’t go through anymore. It is refracted so much that it reflects back in. Demo: light beam on prism, fiber optic strand Critical Angle – it is the angle at which total internal reflection occurs, when the refracted angle is 90o. air glass only occurs when light travels from higher to lower indices of refraction. Total Internal Reflection – occurs when the light rays in a more dense material is refracted back into the material it creates a perfect reflection without energy loss applies to mirages and sunsets: Demo: mirage images What are mirages? Optical illusions created by total internal reflection in air. What creates them? A large difference in temp. air (different density) Video: Light Speed Assignment Ch. 29: 26, 27 91 Chapter 28 Highlights: Color and Light Addition Color Subtraction Key Terms Color by Reflection Color by Subtraction Complementary Colors Color by Transmission 92 Class Activity: Draw and Color a picture on white construction paper using only these colors: Red, Blue, Green, What happens when the pictures you made are viewed under specific colors of light? Reds turn black under green light, Greens turn black under red light. What are the colors that make up white light? Red orange yellow green blue indigo violet cyan blue When we see something that’s white what do we actually see? All the colors being reflected Why do we see color? Three color frequency sensitive cone cells in our eyes. High freq. – blue, Mid. freq. – Green, Low freq. – Red Color by Reflection – the color seen is that color being reflected to our eyes, more than one color frequency can be reflected and will be combined to produce other perceived colors Is Black a color? No, it is the absorption of color. What colors make up a color TV set? Why would they be those specific colors? Red, Green, Blue. They correspond to the color freqs. our eyes are sensitive to. Red Green Blue Demo: primary light colors on white board Observe the combinations of the primary colors using the shadows on the white board What colors are made when mixing the Primary Light Colors? Cyan, Magenta, Yellow 93 Magenta Yellow Cyan Magenta = __red__ + __blue__ Yellow = __red___ + ___green___ Cyan = __blue___ + ___green__ White = __red__ + __blue__ + ___green___ Complimentary Colors – 2 colors when combined create white light Blue + __yellow___ = White Red + __cyan___ = White Green + __magenta__ = White Demo: Color Wheel 94 Make a rainbow by mixing only the 3 colors Yellow, Magenta, and Cyan colored ink. Demo: mixing colored paints Color by Subtraction – color pigments will absorb one specific primary color frequency and reflect 2 primary light colors Magenta Magenta Absorbs green Yellow Reflects red and blue Yellow blue red and green Cyan red blue and green Red Red Absorbs green and blue Green Reflects red Green blue and red green Blue red and green blue Cyan Blue 95 Color by Transmission – similar to color by subtraction but its color is the frequencies allowed to shine through the transparent material. Demo: Colored Overheads, Colored News Print Why do we see a Red apple as Red, and Green grass as Green? It reflects red and absorbs blue and green. It reflects green and absorbs blue and red. What are the Primary light colors that are reflected from a yellow dandelion? Red and Green What are the Primary light colors that are reflected from a deep pink flower? Red and Blue What are the Primary light colors that are reflected from a blue car? Blue What Primary pigment colors are combined to make the blue paint for the car? Cyan and Magenta Assignment Ch. 28: 1,2,5-15, 25-37 96 97 Write down key terms with their definitions for each chapter. Write down the equations used in each chapter. Solve the following example questions: 1. What are photons? 2. What is the speed of light in a vacuum? 3. What creates all types of light? 4. Identify, in order from longest wavelength to shortest wavelength, the 8 main forms of electromagnetic radiation on the spectrum. 5. Name one use or origin of each of the different kinds of light from #4. 6. How did Olaus Roemer prove that light has speed? 7. Explain how Michelson calculated the speed of light. 8. List three examples of what is considered a transparent material. Explain the physical property that makes a material transparent. 9. List three examples of what is considered an opaque material. Explain the physical property that makes a material opaque. 10. Why can light be polarized and sound can not? 98 11. What happens when a polarizer is turned 90 degrees relative to another polarizer? 12. Is light reflected from flat surfaces polarized? 13. Given the speed of light, 300,000,000 m/s, how long will it take light to travel from the earth to an asteroid, which is 138,000,000,000 m away? 14. Given the speed of light, 300,000,000 m/s, calculate the wavelength of a radio station that broadcasts at 102.1 Mhz. M = 1,000,000 Hz 15. Explain the physical property that makes smooth metal surfaces good mirrors. 16. What is the law of reflection? 17. Explain the difference between reflection and refraction. 18. What is dispersion? 19. What does the term total internal reflection have to do with refraction? 20. What is one of the main technological uses for the principle of total internal reflection? 99 21. Which one of these materials has the highest index of refraction: water, glass, diamond, air, or plastic? Which one does light travel the slowest? 22. Identify the three primary light colors. 23. Explain, biologically, why these are the three primary light colors. 24. Identify the primary light colors that are reflected off of the following objects and those that are absorbed: yellow dandelion, red apple, green grass, deep pink flower 25. Identify the primary pigment colors. Explain what primary colors are mixed to produce the secondary pigment colors. 100 Chapter 30 Highlights: Flat mirrors Curved mirrors Lenses Key Terms Lens Virtual Image Principal Axis Ray Diagram Convex Mirror Convex Lens Real Image Magnification Focal Point Concave Mirror Concave lens 101 How do mirrors work? They absorb and reradiate light due to the loosely bound outer electrons What happens when you view at a window from inside your house at night? Why does it do this? There is a partial reflection due to the smooth glass surface. The rest goes through. What is the light ray model for light? Light travels in straight lines – particle property of light What happens when light encounters matter? absorbed – the atoms will resonate at the light freq. and turn into heat. transmitted – the atoms absorb and transfer the light from atom to atom through the substance – not in resonance reflected – atoms absorb and reradiate the light at the same incident angle – law of reflection due to the loose outer electrons Demos: laser on mirror, light ray box on mirror Law of Reflection – the incident light ray angle is equal to the reflected light ray angle as measured relative to the normal. Normal – the imaginary perpendicular line to the surface where the light ray strikes the mirror. parallel light rays reflect in a specific way Two kinds of reflections: Regular – light stays parallel, creates a shiny mirrored surface. Diffuse – when light reflects it is scattered in all directions, light that is parallel doesn’t stay parallel when reflected 102 Identify the angle of incidence, angle of reflection and the normal in the diagram: Images – is where light rays converge or appear to converge, lenses or mirrors create images 2 types: 1. Virtual Images – are light rays that appear to converge behind a mirror or lens, you have to look into it to see the image 2. Real Images – are light rays that converge in front of a mirror or lens, images that can be projected onto a screen Magnification – it is a number that relates how much an image is enlarged or reduced relative to the original object. Images can be: Erect – can be in the same direction as the object, an upright image. Inverted – can be flipped upside-down What kind of images do plane mirrors produce? Virtual, upright, M =1 103 What happens when you view yourself in the large concave mirror? When it is close enough you see a virtual, enlarged image. Why do they produce images like these? A curved mirror reflects light in front of the mirror creating real images – a real focal pt. What happens when the hovercraft is sent down the hallway toward the curved end? The hovercraft will bounce off of the curved wall and go through the same point on the normal axis. Recall: the Law of Reflection – light reflects at equal angles Demo: concave mirror, hovercraft at the end of the hall r f C Principal Axis C C = center of curvature (circle radius) f = focal point Principal Axis – the imaginary perpendicular line to the surface of the curved mirror that goes through the center of curvature, and focal point. Focal Point – the point where light rays converge to form real images. Equation for focus: 2 f = C 104 Every point on an object reflects light in all directions and those light rays are reflected from a mirror. It is easier to simplify the object images by applying one of the properties of light rays coming from an object. You need only 2 light rays to determine the properties of the images from the object: Principles for Drawing Ray Diagrams Light Rays travel in straight lines from all points Parallel Light Rays will converge at the focal point Light Rays that go through the focal point will reflect as parallel lines A light ray will travel through the center of curvature to the same point on the object’s image. 105 Identify the images produced in each of these situations, whether they are real or virtual, erect or inverted, enlarged or reduced: a. If the object (O) is outside of the C: Image properties: inverted, reduced (M<1), real C f b. If the O is between the C and f: Image properties: inverted, enlarged (M>1), real C f c. If the O is inside the f: Image properties: erect, enlarged (M>1), virtual C f Demos: images with concave mirror 106 What is a convex mirror? A mirror that bulges outward in the center, used as security mirrors, and for viewing vehicle blind spots. Demo: convex mirror Why do they produce images like these? These curved mirrors have a virtual focal length. Properties Light Focal Forms of Convex Mirrors: rays always diverge point is behind the mirror therefore, focal point is always = -f images smaller than normal and are virtual images. Follow the same ray diagram rules to find the properties of the images formed by convex mirrors: Image properties: virtual, upright, reduced (M<1) f C 107 What is a lens? A curved piece of transparent material that varies in thickness What are they used for? Bending light rays toward or away from a focal point What are they made out of? Any refracting material, but can be plastic or glass Lens – a curved piece of transparent material that focuses light rays Lenses have two focal points on either side Two kinds of lenses: 1. Convex – thicker in the middle and focuses light rays Bends light inward – has a focus Concave – thinner in the middle and diverges light rays Bends light outward – always disperses and only creates virtual images. 108 The ray diagrams for lenses are very similar to those drawn for spherical mirrors. However, the light rays are not reflected but refracted through the focus on the opposite side of the lens. p h h’ q Principles for Drawing Ray Diagrams for Lenses Light Rays travel in straight lines from all points Parallel Light Rays will converge at the focal point Light Rays that go through the focal point will refract as parallel lines A light ray that travels through the center of the lens will travel in a straight line. 109 Identify the properties of a convex lens: Demo: convex and concave lenses 1. 2f f f 2f 2f f f 2f 2f f f 2f 2. 3. 110 Identify the properties of a concave lens: 1. 2f f f 2f 1. What type of lens can be used to burn holes in paper using sunlight? 2. What type of lens can’t be used to burn holes in paper using sunlight? 3. Do convex lenses with thick centers have short or long focal lengths versus convex lenses that are thinner? Why? Assignment Ch. 30: 1-3, 7,8, 22-30, 36 111 Purpose: To calculate the focal lengths of various lenses by direct measurement. Theory: The focal length of a lens can be measured based on the object and image positions measured relative to the lens. The light from a light bulb will be the object. It will need to be far from the location of the lens as this will cause the light rays from the bulb to be as close to parallel as possible. Using the property of lenses, where parallel light rays converge at the focal point, the focal length will be measured based on this distance. Set-up/Procedure: Lens Meter stick stand Light bulb from across the room Screen Given the set-up illustrated above: 1. Place the lens at a convenient location on the meter stick (ex. 20 cm mark). 2. Slide the screen back-and-forth on the meter stick until a sharp image of the lightbulb is formed. Pay particular attention to the image of the light bulb because it will be very small on the image formation. 3. Measure the image distance from the screen to the lens. This will be the focal length of the lens. 4. Repeat this process for each of the remaining lenses Measure the focal length of each of the 6 numbered lenses. Also use two of lenses from the extras, and find their focal length. Lens 1 2 3 4 5 6 Focal Length (cm) 112 Name: Period: Write down key terms with their definitions for each chapter. Write down the equations used in each chapter. Solve the following example questions: 1. Distinguish between a convex and concave lens and mirrors. 2. What is a focal point? 3. How many rays are used to determine where an image will be located in a ray diagram? Describe which ones. 4. What types of images are formed by convex lenses? Concave? 5. How could you demonstrate that an image is truly real? 6. What type of lens can be used to fry ants? 7. What type of lens exists in your eye? 8. Draw a ray diagram of a convex lens. 9. In which cases will light undergo a greater change in speed? Traveling from air into water, or water into glass? 113 10. Where should you set the focus on your camera if you wanted to take a picture of yourself in a plane mirror standing 2m in front of the mirror? 11. What kind of lens is often included in emergency survival packs? Why? 12. What direction is the image formed by a convex lens - upside-down or right-side up? What direction is the image formed on the retina of your eye? 13. What is a virtual and real image? How can you tell the difference? 14. Would the lens of a camera still work if the speed of light in air was the same as the speed of light in the glass lens? Explain. 114 Name: Period: Write down key terms with their definitions for each chapter. Write down the equations used in each chapter. Solve the following example questions: 1. Work is required to lift a heavy barbell. How many times more work is necessary to lift it 3 times as high? 2. If a barbell weighs 120 N, and it is lifted a distance of 2 m, how much work was required? If it were lifted a distance of 6m? 3. What if lifting the barbell in #2 was done in a time of 3s, how much power was developed in both cases? 4. A 50 kg object has a velocity of 10m/s, what is it’s kinetic energy? 5. If the previous object is traveling twice as fast, how much kinetic energy will it possess? Three times as fast? 6. A 10 kg rock on the edge of a 100 m cliff has how much potential energy? How much kinetic energy will it have right before it strikes the ground if it were to fall. How fast will it be traveling? 115 7. What is the mechanical advantage of a machine that applies a force of 800N when a force of 80 N is applied to lift a heavy weight? If an input distance of 1m was applied to the machine, how high was the weight lifted? 8. What is the relationship between the MA and the ropes on a pulley? 9. What is the mechanical advantage of a ramp that is 10m long that has a vertical distance of 2m? 10. What would the amount of force required to lift a 500 N object using the ramp in #10? Assume there is not friction. 11. If friction were present in the ramp from the previous example and it required 150 N of force to push it up the ramp, what is the efficiency of the ramp? 12. What is used to measure temperature? 13. Convert 74 degrees F, into Celsius, and Kelvin. 14. Do substances that normally heat up quickly have a high or low specific heat? 116 15. Compare the specific heat of water to other known substances, how does it compare? Why? 16. Why does a bimetallic strip bend when heated or cooled? 17. Which of these expand the most when heated: Solids, Liquids, Gases? Why? 18. At what temperature is the density of water the greatest? Why? 19. Calculate the number of heat calories when 400g of water is heated from 20 degrees Celsius to 80 degrees. 20. Calculate the number of heat calories lost by a 1200 g chunk of copper metal, c = 0.2, as it cools from 100 degrees to 30 degrees. 21. What are the three forms of heat transfer? Explain each. 22. What color is a good absorber of heat energy? What is a good emitter? 23. What reduces the efficiency of a heat engine? Use Carnot’s efficiency as a guide. 24. If an engine is frictionless, is it 100% efficient? 117 25. Calculate the max. efficiency of an engine that operates between a high temp. of 900 K and a low temp. of 300 K. 26. Draw a sine curve and label the following: Amplitude, crest, trough, wavelength, neutral line. 27. As the frequency of sound increases what happens to the wavelength? 28. Describe the two different kinds of interference and what creates them. 29. Draw a 1 wavelength standing wave and label the following parts: anti-node, node. 30. The Sears tower in Chicago sways back and forth with a period of 8 seconds. What is the frequency of vibration? 31. Calculate the speed of a wave if the crests are 0.3 m apart and two crests pass by a stationary point in 2 seconds. 118 32. Calculate the wavelength of a 200 Hz sound in air, assume the speed of sound is 340 m/s. 33. Explain the Doppler Effect. Does the Doppler effect occur for some types of waves or all types of waves? 34. The frequency of a favorite radio station is 103.1 MHz. What would be the wavelength of this radio signal? The speed of light is 3 x 108 m/s. 35. What is the source of all sounds? 36. Compare the speed of sound in air to the speed of sound in water and metals. Explain their difference. 37. What is the average frequency range of human hearing? 38. Calculate the wavelength range of human hearing. 39. What kind of wave is a sound wave? 40. What effect does using a sounding board on a musical instrument have on the sound it produces? Explain why this works. 41. What happens when a musical instrument produces resonance? 42. How is the amount of sound from a source measured? 119 43. A 10 dB sound is made. Comparatively, how much more intense is a 20 dB sound? 30 dB? 40 dB? 44. Suppose you have a 512 Hz tuning and you hear a 12 Hz beat frequency when sounded with another unknown tuning fork. What are the two possible frequencies of the unknown tuning fork? 45. What are photons? 46. What is the speed of light in a vacuum? 47. What creates all types of light? 48. Identify, in order from longest wavelength to shortest wavelength, the 8 main forms of electromagnetic radiation on the spectrum. 49. Name one use or origin of each of the different kinds of light from #48. 50. List three examples of what is considered a transparent material. Explain the physical property that makes a material transparent. 51. Explain the physical property that makes a material opaque. 120 52. Explain the difference between polarized and non-polarized light. 53. Why can light be polarized and sound cannot? 54. What happens when a polarizer is turned 90 degrees relative to another polarizer? 55. Is light reflected from flat surfaces polarized? 56. Given the speed of light, 300,000,000 m/s, how long will it take light to travel from the earth to an asteroid, which is 138,000,000,000 m away? 57. Identify the three primary light colors. 58. Identify the primary light colors that are reflected off of the following objects and those that are absorbed: yellow dandelion, red apple, green grass, deep pink flower. 59. Identify the primary pigment colors. Explain what primary colors are mixed to produce the secondary pigment colors. 60. Explain the physical property that makes smooth metal surfaces good mirrors. 61. What is the law of reflection? 121 62. Explain the difference between reflection and refraction. 63. What is dispersion? 64. What does the term total internal reflection have to do with refraction? 65. What is one of the main technological uses for the principle of total internal reflection? 66. Which one of these materials has the highest index of refraction: water, glass, diamond, air, or plastic? Which one does light travel the slowest? 67. Identify the image properties when an object is placed at the following distances from a convex lens: (real/inverted; enlarged/reduced; erect/inverted) a. Greater than 2f b. Less than f c. Between f and 2f 68. Identify the image properties when an object is placed at the following distances from a concave lens: : (real/inverted; enlarged/reduced; erect/inverted) a. Greater than 2f b. Less than f c. Between f and 2f 122 69. Distinguish between a convex and concave lens and mirrors. 70. What is a focal point? 71. How many rays are used to determine where an image will be located in a ray diagram? Describe which ones. 72. What types of images are formed by convex lenses? Concave? 73. How could you demonstrate that an image is truly real? 74. What type of lens can be used to fry ants? 75. What type of lens exists in your eye? 76. Draw a ray diagram of a convex lens. 77. In which cases will light undergo a greater change in speed? Traveling from air into water, or water into glass? 78. Where should you set the focus on your camera if you wanted to take a picture of yourself in a plane mirror standing 2m in front of the mirror? 123 79. What kind of lens is often included in emergency survival packs? Why? 80. What direction is the image formed by a convex lens? Upside-down or right-side up? What direction is the image formed on the retina of your eye? 81. What is a virtual and real image? How can you tell the difference? 124