(SM-NZVI) in Saturated Porous Media: Effects of Surface Stabilizer
advertisement

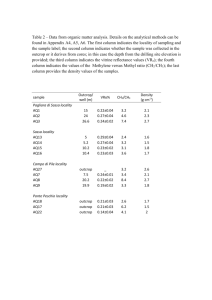
Supporting Information Transport of Surface-Modified Nano Zero-Valent Iron (SM-NZVI) in Saturated Porous Media: Effects of Surface Stabilizer Type, Subsurface Geochemistry and Contaminant Loading Haoran DONG1,2 and Irene M. C. LO1* 1 Department of Civil and Environmental Engineering, The Hong Kong University of Science and Technology, Hong Kong, China 2 College of Environmental Science & Engineering, Hunan University, Changsha, Hunan, China *Corresponding author: Email: cemclo@ust.hk; 852-23587157. − Pages: 1-10 − Figures: Fig. S1-S8 − Table: Table S1 1 Fax: 852-23581534; Tel: Preparation and characterization of SM-NZVIs Two commercial NZVIs in aqueous dispersion form were supplied by the NANOIRON® Company (Czech Republic, EU): pristine NZVI (Nanofer 25, produced from nanosized ferrihydrite) and surface coated by PAA (Nanofer 25S). Nanofer 25 (referred to as CNZVI in this study) was used for further modification by using Tween-20 or starch. Information on the three surface stabilizers is summarized in Table S1. Deoxygenated ultrapure water was used for the preparation of SM-NZVIs to avoid the oxidation of Fe0 during the modification process. PAA-modified CNZVI (i.e., Nanofer 25S), Tween-20-modified CNZVI and starch-modified CNZVI are referred to as P-CNZVI, T-CNZVI and S-CNZVI, respectively, in the following. T-CNZVI was prepared by dispersing CNZVI particles in aqueous Tween-20 to result in suspensions comprising iron nanoparticles (1.0 g L-1) and Tween-20 (35 wt%), followed by sonication for 30 min. The method of preparation of S-CNZVI is as described below. Briefly, a 0.4 wt% starch solution was prepared by mixing 0.8 g of potato starch with 200 mL of ultrapure water and heating the mixture to 100 oC. Once the starch solution started boiling, the heating was removed and the solution was allowed to cool at room temperature. The cooled starch stock solution was then introduced into CNZVI stock suspensions to result in suspensions comprising iron nanoparticles (1.0 g L-1) and starch (0.4 wt%), followed by sonication for 30 min. The SM-NZVI suspensions were freshly prepared before each experiment. Mössbauer spectroscopy confirmed that ~85% were in zero-valent state in CNZVI, and no 2 obvious loss of Fe0 was observed during the surface modification. Morphological analysis of NZVI particles was performed by TEM (JEOL 2010 TEM). The individual particles of P-CNZVI, T-CNZVI and S-CNZVI appear spherical and have an average diameter of about 7 nm, 9 nm and 4 nm, respectively. The hydrodynamic particle sizes of P-CNZVI, T-CNZVI and S-CNZVI (Measured at 100 mg L-1) were 183 nm, 136 nm and 361 nm, respectively, and were determined by using dynamic light scattering (DLS) (Zetaplus, LaborScience S.A.). Aggregation of the nanoparticles with time was monitored by measuring the time-dependent hydrodynamic diameter via DLS. Zeta potential measurement of porous media The streaming potential of the porous medium (sand and soil) was measured using an Electro Kinetic Analyzer (Anton Paar GmbH, Graz, Austria) equipped with a cylindrical cell to house the granular porous medium. Sand or soil after saturation with various flushing solutions was wet-packed into the cylindrical cell in a solution as same as the flushing solution used during the corresponding column experiments. Before the start of each measurement, the cell was equilibrated by circulating the solution for 20 min. Streaming potentials were converted to zeta potentials using the Helmholtz–Smoluchowski equation. Determination of As(V) sorption on T-CNZVI Sorption kinetics experiments were conducted using a 100 mg L-1 T-CNZVI suspension and 1 mg L-1 As(V) in synthetic groundwater at pH 7. The final solutions (40 mL) in 41-mL glass vials sealed with Teflon caps were shaken in an end-over-end 3 rotator at 26 rpm, at room temperature (25 ºC). At pre-determined time intervals, suspensions were filtered using 0.2-μm pore size cellulose nitrate filters. It should be noted that all three types of nanoparticles had particle sizes smaller than 200 nm before the reaction and could possibly pass through the filters; thus the iron concentration in the filtered solution was analyzed. However, the results showed that the concentrations of iron were non-detectable, indicating that all the SM-NZVI could be retained by the filter. This should be ascribed to the formation of larger aggregates (d > 200 nm) after the reaction with As(V). The filtrates were acidified, diluted (if necessary) and analyzed using a Graphite Furnace-Atomic Absorption Spectrophotometer (GF-AAS) (Hitachi, Z-8200) for total arsenic concentration. The results are shown in Fig. S2 and the equilibrium time for As(V) adsorption by T-CNZVI was determined to be 120 min. As(V) sorption capacity of T-CNZVI was then evaluated. A range of As(V) (0-20 mg L-1) was reacted with T-CNZVI (100 mg L-1) in the synthetic groundwater. After reaching the adsorption equilibrium, the T-CNZVI suspensions were filtered and the arsenic concentration in the filtrate was measured. As the predominant mechanism of As(V) removal by NZVI is adsorption onto the NZVI corrosion products (Kanel et al., 2006), the adsorption isotherm for the As(V) adsorption on T-CNZVI was examined and the plots are shown in Fig. S3. A Langmuir adsorption isotherm (Eq. 1), was able to describe As(V) adsorption by T-CNZVI. qe = qmax αCe / (1+αCe) Eq. 1 4 where qe is the amount of As sorbed (mg g-1), α (L mg-1) is a parameter related to the affinity of the sorbent for the sorbate, qmax (mg g-1) is the maximum As sorption capacity, and Ce is the equilibrium As concentration in the solution (mg L-1). For T-NZVI, the equation (R2 > 0.992) gives values of qmax (13.9 mg g-1 at pH 7). Fig. S1 Schematic of column experiments for the transport of SM-NZVI 100 Arsenic removal (%) 80 60 40 20 0 0 100 200 300 400 500 Time (min) Fig. S2 Kinetics of arsenic removal by T-CNZVI at pH 7 (Fe0=100 mg L-1, As(V)=1 mg L-1). 5 12 0.9 a 0.7 8 Ce/qe qe(mg/g) b 0.8 10 6 4 0.6 0.5 0.4 2 As(V) removal by T-CNZVI Fit curve 0.3 0 0.2 0 2 4 6 8 10 0 2 4 Ce (mg/L) 6 8 10 Ce (mg/L) Fig. S3 Adsorption isotherm plots (a) and Langmuir adsorption plots (b) for the adsorption of As(V) on T-CNZVI at pH 7 (Fe0=100 mg L-1). 30 20 10 a2 a3 P-CNZVI mass ratio (%) -1 HA=0 mg L , Sand HA=10 mg L-1, Sand HA=0 mg L-1, Soil HA=10 mg L-1, Soil P-CNZVI mass ratio (%) a1 40 P-CNZVI mass ratio (%) P-CNZVI mass ratio (%) 50 a4 0 9 12 15 3 6 9 12 15 b2 HA=0 mg L-1, Sand HA=10 mg L-1, Sand HA=0 mg L-1, Soil HA=10 mg L-1, Soil 30 20 10 6 9 12 15 3 Column section (cm) S-CNZVI mass ratio (%) b1 40 3 Column section (cm) S-CNZVI mass ratio (%) S-CNZVI mass ratio (%) 6 Column section (cm) b3 6 9 12 15 Column section (cm) S-CNZVI mass ratio (%) 3 50 b4 0 9 12 15 3 HA=0 mg L-1, Sand HA=10 mg L-1, Sand HA=0 mg L-1, Soil HA=10 mg L-1, Soil 30 20 10 9 12 15 3 c2 6 9 12 15 3 Column section (cm) T-CNZVI mass ratio (%) c1 40 6 Column section (cm) T-CNZVI mass ratio (%) T-CNZVI mass ratio (%) 6 Column section (cm) c3 6 9 12 15 Column section (cm) T-CNZVI mass ratio (%) 3 50 c4 0 3 6 9 12 Column section (cm) 15 3 6 9 12 15 Column section (cm) 3 6 9 12 Column section (cm) 15 3 6 9 12 Column section (cm) Fig. S4 Distribution of (a) P-CNZVI; (b) S-CNZVI and (c) T-CNZVI along the length of column at the end of injection: (1) DI water system with 100 mg L-1 of Fe0; (2) Synthetic groundwater system with 100 mg L-1 Fe0; (3) DI water system with 1 g L-1 of Fe0 and (4) Synthetic groundwater system with 1 mg L-1 Fe0. Note: Column sectioning is from the inlet to the outlet. In X-axis, “3” represents the column section of “0-3 cm” and “6” represents the column section of “3-6 cm”, etc. 6 15 500 600 -1 HA=0 mg L (DI) HA=10 mg L-1 (DI) HA=0 mg L (GW) HA=10 mg L-1 (GW) Radius (nm) 400 Radius (nm) -1 300 500 400 200 a 100 b 300 0 10 20 30 40 50 60 0 Time (min) 10 20 30 40 50 60 Time (min) Fig. S5 Aggregation of P-CNZVI in DI water and synthetic groundwater (GW) in the absence and presence of HA. (a) Fe0=100 mg L-1; (b) Fe0=1 g L-1 7 DI 0 HA GW GW+HA GW GW+HA Zeta potential (mV) -10 -20 -30 -40 X Data -50 a Zeta potential (mV) 0 DI HA -5 -10 -15 Zeta potential (mV) 0 b -5 -10 -15 c -20 Fig. S6 Zeta potential at pH 7 of (a) P-CNZVI, (b) T-CNZVI and (c) S-CNZVI in DI water and synthetic groundwater (GW) in the absence and presence of HA. Error bars represent the standard deviations of duplicate experiments 8 Fig. S7 TEM images of P-CNZVI in the presence of HA (10 mg L-1): (a) P-CNZVI=100 mg L-1; (b) P-CNZVI=1 g L-1 300 500 a T-CNZVI (Fe0=100 mg L-1) c S-CNZVI (Fe0=100 mg L-1) 250 Radius (nm) Radius (nm) 450 200 150 HA=0 mg L-1 (DI) HA=0 mg L-1 (GW) HA=10 mg L-1 (DI) HA=10 mg L-1 (GW) 100 50 c 400 350 0 300 0 10 20 30 40 50 60 0 10 20 30 Time (min) 600 700 0 T-CNZVI (Fe =1 g L ) 0 60 -1 S-CNZVI (Fe =1 g L ) 600 Radius (nm) 500 Radius (nm) 50 d b -1 40 Time (min) 400 300 500 400 200 300 0 10 20 30 40 50 0 60 10 20 30 40 50 60 Time (min) Time (min) Fig. S8 Aggregation of (a, b) T-CNZVI and (c, d) S-CNZVI in DI water and synthetic groundwater (GW) in the absence and presence of HA. 9 Table S1 Three types of surface stabilizers for modification to NZVI Surface-Modified NZVI Category of Surface Stabilizer Molecular Structure of Stabilizer Stabilization Mechanism P-CNZVI PAA (Polyelectrolytes)1 Electrosteric T-CNZVI Tween-20 (Non-ionic Surfactant)2 Steric S-CNZVI Starch (Hydrophilic biopolymers)3 Steric 1 Hydutsky et al., 2007 Kanel et al., 2007 3 He and Zhao, 2005 2 References (1) Hydutsky, B. W., Mack, E. J., Beckerman, B. B., Skluzacek, J. M., Mallouk, T. E., 2007. Optimization of nano- and microiron transport through sand columns using polyelectrolyte mixtures. Environmental Science & Technology 41, 6418–6424. (2) Kanel, S. R., Nepal, D., Manning, B., Choi, H., 2007. Transport of surface-modified iron nanoparticle in porous media and application to arsenic(III) remediation. Journal of Nanoparticle Research 9, 725–735. (3) He, F., Zhao, D., 2005. Preparation and characterization of a new class of starch-stabilized bimetallic nanoparticles for degradation of chlorinated hydrocarbons in water. Environmental Science & Technology 39, 3314–3320. 10