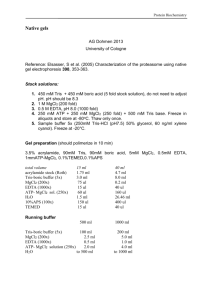
Molarity A measure of the concentration of a solution in moles per
advertisement

Molarity A measure of the concentration of a solution in moles per Liter solution. A solution is a homogeneous mixture in which the particle size is so small that it cannot be seen with a microscope and is uniformly distributed throughout the solution. A solution is made of a solute dissolved in to a solvent. A solute is the substance that is dissolved into the solvent. For example in a solution of salt water, the salt is the solute and the water is the solvent. A solution may be a solid dissolved in a liquid, a liquid dissolved in a liquid, a gas dissolved in a liquid, a gas dissolved in a gas. Molarity is represented by a capital M. M= mol/L Molarity is moles divided by Liters Example problems: 1. Find molarity of a solution that has 3g MgCl2 dissolved in 50mL water. 3g MgCl2/95g/mol MgCl2 = 0.0315mol MgCl2 0.0315 mol MgCl2/0.05L = 0.632 M MgCl2 2. A student has 190mL of 1.1M MgCl2 solution. Find the mass of MgCl2 dissolved in solution. M(L)= mol (1.1)(0.19)=0.209 mol MgCl2 0.209 mol MgCl2 x 95g/mol MgCl2 = 19.86g MgCl2 3. How many liters of solution can be made of a 2M solution using 80g NaOH? mol/M= L 80g NaOH/40g/mol NaOH = 2mol NaOH 2mol NaOH/2M = 1L solution Dilution Problems: To dilute a solution is to make it less concentrated by adding more solvent. Often times in chemistry we need to make a dilute solution from a highly concentrated original solution. The equation is as follows: M1V1=M2V2 Where M1 and V1 are the molarity and volume of the original solution and M2 and V2 are the molarity and volume of the dilute solution. Example: 1. How would you prepare 2L of a 0.25M NaOH solution from 1M stock solution? *the stock solution is the more concentrated solution you have in stock or that you are starting with. You are figuring out how much of the concentrated solution to pour into a beaker and then how much water you will add to dilute it to the concentration and volume you want. M1V1=M2V2 (1M)(V1) = (0.25M)(2L) V1= 0.5L Start with 0.5L of 1MNaOH and add 1.5L of water (The amount of water added is the difference between the amount of original solution you start with and the total volume of solution. In this case take the 2L of final solution and subtract the 0.5L you will start with in the beaker. Therefore 1.5L of water will be added to the 0.5L of 1M NaOH in the beaker.)