tpj12913-sup-0014-AppendixS1
advertisement

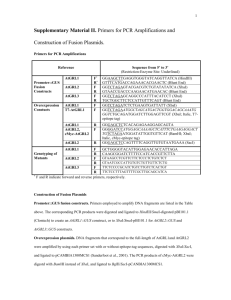
Methods S1: Vector constructions and transformation of yeast and fungal isolates pBIN, a vector suitable for transient expression experiments, was used to express AvrLm4-7 in tobacco leaves, alone or fused to eGFP. eGFP coding sequence was amplified from plasmid peGFP (Clontech, Mountain View, CA, USA) using primers pBINeGFPXbaUp (which introduces a XbaI restriction site) and pBINeGFP-SacLo (which introduces a SacI restriction site). eGFP PCR product was digested by XbaI and SacI and ligated into XbaI-SacI digested pAG1 (Bourreau et al., 2006). AvrLm4-7 fragment without signal peptide was amplified from cDNA of v23.1.3 mycelium using respectively primers pBINAvr47noPSXbaUp (which introduces a XbaI restriction site) and pBINAvr47noStop-XbaLo or pBINAvr47Stop-XbaLo (which introduces also a XbaI restriction site). The PCR products were digested by XbaI and ligated into XbaI digested pBIN-eGFP or pBIN. The constructs, termed pBIN-A47noPS-eGFP and pBIN-A47noPS were cloned in E. coli, reextracted and sequenced. Finally, the constructs were introduced into A. tumefaciens strain C58 by electroporation at 1.5 kV, 200 ohms and 25 µF. A. tumefaciens strain C58 containing carrying pBIN-eGFP construct was used as control of efficient transient expression in tobacco leaves. The vector pPIC9 (Invitrogen, Cergy Pontoise, France) was used to express AvrLm47, without signal peptide and with an histidine-tag in the C terminal part of the protein, in P. pastoris. AvrLm4-7 fragment was amplified from cDNA of v23.1.3 or v23.1.2 mycelium using primers PIC9-A47-Up (which introduces a SnaBI restriction site) and PIC9-A47-Lo (which introduces a NotI restriction site and a sequence encoding for a hexa-histidine tag). AvrLm4-7 PCR product was digested by SnaBI and NotI. AvrLm4-7 fragment was ligated into SnaBI-NotI digested pPIC9. The construct, termed pPIC9-A47, was cloned in E. coli, reextracted and sequenced. Preparation of yeast electrocompetent cells and transformation of P. pastoris GS115 were performed as described by the supplier (Invitrogen, K1710-01). pPIC9-A4-7 plasmid was extracted from E. coli and digested with SalI, and the linear plasmid DNA (5 μg) was transformed into 80 μl of competent GS115 cells by pulsed electroporation using a BTX electro cell manipulator (1,500 V, 25 µF, 200 ohms). Transformed cells were incubated on minimal dextrose (MD) plates at 30 °C for 4–6 days until colonies appeared. Phenotyping of transformants was performed by patching the colonies in replica-plating on minimal dextrose vs. minimal methanol plates. PCR was used to confirm the positive yeast recombinants containing AvrLm4-7. AvrLm4-7 and its mutated versions M1 (RAWG motif mutated to qAWG), M2 (HRYRE motif mutated to HqYqE) and M3 (RAWG and HRYRE motif mutated to qAWG and HqYqE respectively) were synthesized by GenScript (Piscataway, NJ, USA) with codon optimization for expression in E. coli and introduced in vector pUC57. Constructs were then transferred into pDONR (Invitrogen, Cergy Pontoise, France) and finally transferred into other vectors as needed. sAvrLm4-7 constructs were secreted via a soybean PR1a leader (Anderson et al., 2012) while mAvrLm4-7 constructs lacked a secreted leader sequence. For flow cytometry experiments, native AvrLm4-7 and the three mutant genes were ligated into pGAPz-Alpha, in frame with the S. cerevisiae mating factor signal sequence followed by C-terminal myc epitope and hexa-histidine tags. Linearized pGAPz-Alpha containing the respective gene of interest was transformed into P. pastoris strain GS115 and selected under 100 ug/mL Zeocin. The binary vector pPZPNat1, which carries the nourseothricin acetyltransferase nat1 gene (conferring resistance to the antibiotic nourseothricin) under control of the A. nidulans trpC promoter as a selectable marker, was used for A. tumefaciens-mediated transformation of L. maculans. Vector pPZPNat1-A4A7 which carries v23.1.3 allele of AvrLm4-7 and vector pPZPNat1-a4A7 which carries v23.1.2 allele of AvrLm4-7 were as described by Parlange et al. (2009). PCR-mediated site-directed mutagenesis was performed on these two constructs. Using the pPZPnat1-a4A7 construct as template, the primers MD1-Up (with a C299 instead of G299), MD2-Up (with a C305 instead of T305) or MD3-Up (with a A336 instead of C336) were used in combination with primer AVR47-JN2-Lo (Table S1) in a PCR reaction using the high fidelity DNA polymerase Taq Phusion (Finnzymes) to generate respectively 487-bp fragment MP1, 484-bp fragment MP2 or 452-bp fragment MP3. MP1, MP2 or MP3 was then used as a megaprimer in combination with AVR47-JN2-Up in a Taq Phusion PCR reaction to generate 1843-bp products termed v23.1.3-MD1, v23.1.3-MD2, v23.1.3-MD3, v23.1.2-MD1, v23.1.2MD2 and v23.1.2-MD3. Following restriction with SnaBI and BstXI and gel purification, the fragments were used to replace the excised corresponding sequence of pPZPNat1-a4A7. The constructs, termed pPZPNat1-a4A7-MD1, pPZPNat1-a4A7-MD2 and pPZPNat1-a4A7-MD3 were cloned in E. coli, reextracted and accuracy of the point mutations checked by sequencing. The same strategy was adopted to generate mutated versions of KGLI, RAWG and RYRE motifs by using primers RxLRMD1-Up (with a C157 instead of A157), RxLRMD2Up (with a G157 instead of A157), RxLRMD3-Up (with a AA157-158 instead of GT157-158), RxLRMD4-Up (with a AAA157-156 instead of CGT156-158) and RYRE-MD1-Up (with a CA292293 and A299 instead of AG292-293 and G299) and pPZPNat1-A4A7 construct as template. Finally, the constructs were introduced into A. tumefaciens and used for transformation of isolate G07E106 (virulent towards Rlm4 and Rlm7). The constructions were then introduced into A. tumefaciens strain C58 by electroporation at 1.5 kV, 200 ohms and 25 µF. Transformation of L. maculans was performed as described by de Groot et al. (1998) with minor modifications (Gout et al., 2006). Fungal transformants were selected on 50 µg/ml nourseothricin (WERNER BioAgents, Jena, Germany). Anderson, R.G., Casady, M.S., Fee, R.A., Vaughan, M.M., Deb, D., Fedkenheuer, K., Huffaker, A., Schmelz, E.A., Tyler, B.M., McDowell, J.M. (2012) Homologous RXLR effectors from Hyaloperonospora arabidopsidis and Phytophthora sojae suppress immunity in distantly related plants. Plant J., 72, 882-893. Boureau, T., El Maarouf-Bouteau, H., Garnier, A., Brisset, M.N., Perino, C., Pucheu, I. and Barny, M.A. (2006) DspA/E, a type III effector essential for Erwinia amylovora pathogenicity and growth in planta, induces cell death in host apple and nonhost tobacco plants. Mol. Plant Microbe Interact., 19, 16-24. Gout, L., Fudal, I., Kuhn, M.L., Blaise, F., Eckert, M., Cattolico, L., Balesdent, M.H. and Rouxel, T. (2006) Lost in the middle of nowhere: the AvrLm1 avirulence gene of the dothideomycete Leptosphaeria maculans. Mol. Microbiol., 60, 67-80. de Groot, M.J.A., Bundock, P., Hooykaas, P.J.J. and Beijersbergen, A.G.M. (1998) Agrobacterium tumefaciens-mediated transformation of filamentous fungi. Biotechniques, 16, 839-842. Parlange, F., Daverdin, G., Fudal, I., Kuhn, M.L., Balesdent, M.H., Blaise, F., GrezesBesset, B. and Rouxel, T. (2009) Leptosphaeria maculans avirulence gene AvrLm4-7 confers a dual recognition specificity by the Rlm4 and Rlm7 resistance genes of oilseed rape, and circumvents Rlm4-mediated recognition through a single amino acid change. Mol. Microbiol., 71, 851-863.