From: Dr. Ratn Deep Singh Assistant Professor, Veterinary College
advertisement

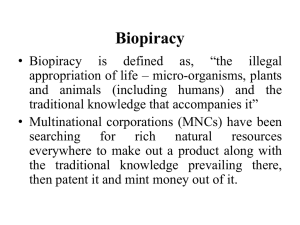
From: Dr. Ratn Deep Singh Assistant Professor, Veterinary College, S.D.A.U., S. K. Nagar, Dantiwada, Gujarat -385506 To, The Editor, International Journal of Research & Development in Pharmacy & Life Sciences Subject: Submission of review article for publication in IJRDPL. Sir, Kindly find herewith a review article entitled “Pharmaceutical Biopiracy and Protection of Traditional Knowledge” authored by R. D. Singh et al., typed in A4 size paper with double space, for publication in IJRDPL. I have furnished below the information regarding complete address and e-mail ID of communicating author and institution. It is to be certified, on the behalf of all authors, that the article has not been published elsewhere in any language or sent for publication in any other journal. The authors of this article agree to all terms and conditions indicated in the ‘Instruction to Authors’. Please acknowledge the receipt of the article and status of publication for the same. Yours sincerely DATE: 05-12-2013 (RATN DEEP SINGH) Title of the review article: Pharmaceutical Biopiracy and Protection of Traditional Knowledge. Name and Address of the corresponding author: Dr. Ratn Deep Singh Assistant Professor Department of Pharmacology & Toxicology College of Veterinary Science & Animal Husbandry Sardarkrushinagar Dantiwada Agricultural University Sardarkrushinagar, Dantiwada, District: Banaskantha State: Gujarat (India) Pin code – 385 506 e-mail ID: ratn1709@yahoo.com Contact no.:- +91-96385 17179 Name and address of all the authors: 1. Ratn Deep Singh (Assistant Professor) 2. Shailesh K. Mody (Professor & Head) 3. Hitesh B. Patel (Assistant Professor) 4. Sarita Devi*(Assistant Professor) 5. Chirag M. Modi (Senior Research Fellow) 6. Divyesh R. Kamani (Postgraduate Scholar) Address: Department of Pharmacology & Toxicology College of Veterinary Science & Animal Husbandry Sardarkrushinagar Dantiwada Agricultural University Sardarkrushinagar, Dantiwada, Banaskantha, Gujarat -385 506 (India) * Department of Medicine College of Veterinary Science & Animal Husbandry Sardarkrushinagar Dantiwada Agricultural University Sardarkrushinagar, Dantiwada, Banaskantha, Gujarat -385 506 (India) PHARMACEUTICAL BIOPIRACY AND PROTECTION OF TRADITIONAL KNOWLEDGE R. D. Singh*, S.K. Mody, H. B. Patel, Sarita Devi, C.M. Modi and D.R. Kamani Department of Pharmacology & Toxicology College of Veterinary Science & Animal Husbandry Sardarkrushinagar Dantiwada Agricultural University Sardarkrushinagar, Gujarat -385 506 (India) KEY WORDS: Traditional knowledge, Biopiracy, CBD, Nayoga Prorocol, TKDL TRADITIONAL KNOWLEDGE According to UNESCO definition, Traditional Knowledge (TK) is “the cumulative and dynamic body of knowledge, know-how and representations possessed by peoples with long histories of interaction with their natural milieu. It is intimately tied to language, social relations, spirituality and worldview, and is generally held collectively”. As defined by the World Intellectual Property Organization (WIPO), it is knowledge, know-how, skills and practices that are developed, sustained and passed on from generation to generation within a community, often forming part of its cultural or spiritual identity [1]. TK includes indigenous knowledge, folklore, and traditional medical knowledge and often used to develop commercial products such as new pharmaceuticals, herbal medicines, seeds, cosmetics, personal care and crop protection products, e.g. traditional medicine may be used to guide the screening of plants for medically active compounds. Knowledge about characteristics of plants having healing properties and technology of its use gives medicinal plants their social and economic value. This technology of use has been acquired through thousands of years of experience, trial and error and generation to generation refinement. As a result of this, age-old communities have developed their knowledge of the plant, animal and mineral resources to a grown-up and scientifically-sound technology, which reflects in old traditions of healing science like Ayurveda and Siddha. In addition to this, tribal, island and local ethnic communities have developed their own knowledge base about the flora, fauna and mineral wealth of their region [2]. BIOPIRACY Biopirates, pirates of life, are pillaging a new kind of wealth, that of biodiversity and the traditional knowledge and techniques of rural and indigenous peoples. Biopiracy can be defined as, “the misappropriation and commercialization of genetic resources and traditional knowledge of rural and indigenous people” [3]. Pharmaceutical biopiracy is a term used generally to describe the legal practice by pharmaceutical companies exploiting the indigenous people’s traditional knowledge of medicine. India and other developing countries are rich in bio-resources and TK are favourite targets and victims of biopiracy. Turmeric, neem and basmati rice were well known examples of biopiracy. Renowned economist and Nobel Prize winner Joseph E. Stiglitz comments on the World Trade Organization’s Trade Related Aspects of Intellectual Property Rights Agreement (TRIPS) - What we were not fully aware of was another danger, what has come to be termed bio-piracy, international companies patenting traditional medicines and foods. It is not only that they seek to make money from ‘resources’ and knowledge that rightfully belongs to the developing countries, but in so doing, they squelch domestic firms that have long provided the products [4]. Biopirates are mainly pharmaceutical, cosmetic and agri-food firms. Biopiracy of genetic resources and genetic materials are also noticed. They draw on biodiversity hot-spots in order to create supposedly “innovative” products and guarantee their monopoly on them through the patent system. Such misappropriation of TK results in grant of patent for the invention to the “first–to–file” (the pharmaceutical company) rather than to the “first–to–invent” (the indigenous community). It involves making profit from freely available natural products (plants, seeds, leaves etc.), by copying techniques used daily for generations by local peoples in order to feed or take care of themselves. Biopirates do not give any profit or proper benefit to local communities and TK holders [3]. BIOPROSPECTING, BIOPIRACY & PHARMACEUTICAL INDUSTRY Bio-prospecting, which usually precedes biopiracy, is the systemic search for, and the development of, new sources of chemical compounds, genes, micro and macro-organisms and other valuable bio-products. One of the biggest threats to biodiversity and related traditional knowledge is ever increasingly bio-prospecting activities on behalf of ethno-botanists, pharmaceutical companies and others who wish to profit from the rich biodiversity and traditional knowledge in indigenous territories [2, 5]. Bio-prospecting is the exploration of biodiversity for new biological resources of social and economic value. It is carried out by a wide variety of industries, the best known being the pharmaceutical industry. Research-based industries have found it profitable to screen natural resources such as soil samples, marine waters, insects, tropical plants and animals in developing countries. Moreover, in recent times, enormous cost of drug development along with growing incidence of side effects and drug-resistance has become of great concern. The search for new molecules is, therefore, expensive, uncertain and runs the risk of huge payments in liability when things go wrong. As compared to the conventional system of screening millions of synthesized chemicals, TK based bio-prospecting may significantly cut costs of pharmaceutical R&D. Hence the Pharma-industry is looking increasingly at medicines and products that have been developed by local communities in older cultures like India, Africa and China where the centuries-old traditions of indigenous healing are still viable and in use. These healing practices and cures are rich hunting grounds for biopirates. Pharmaceutical bio-prospecting has been sharply criticized for what has become known as ‘biopiracy’ in which large international pharmaceutical corporations make use of local indigenous or traditional knowledge, without acknowledging that it is indigenous intellectual property. Thus, profits have accrued solely to the pharmaceutical companies and indigenous peoples received little or nothing in return [6]. The pharmaceutical industry has had a long and fruitful relationship with biodiversity. Large pharmaceutical companies generate close to USD 250 billion annually from drugs directly derived from biodiversity. In 2010, the natural products mix in the pharmaceutical industry was estimated to be 40%. Currently, 62% of cancer drugs approved by the US Food and Drug Administration come from, or are modeled based on, natural products. In 2010, more than 40% of all the new chemical entities were obtained from natural sources. Nearly 48% of drugs in the clinical phase are derived from plants. Drugs currently in the pipeline that are derived from natural sources are mostly cancer and anti-infective medicines. These two therapeutic areas account for 56% of all drugs of natural origin in clinical trials [7]. Collaborations between pharmaceutical companies and the countries supplying the indigenous knowledge and medicinal resources could offer an important new revenue source for impoverished developing countries. Therefore, efforts to establish fair and equitable partnerships between the pharmaceutical industry and the developing countries are imperative to ensure sustainable, mutually beneficial relationships [8]. PATENT IS MAIN TOOL FOR BIOPIRATES Patent law, while largely influenced by western legal concepts, can differ from nation to nation. There has been some movement towards harmonizing patent law to satisfy the needs of many countries, but the interests of industrialized nations remain at the forefront of patent theory. Patent law is affected by competing government interests, but is also influenced by other entities, such as trade organizations and nongovernmental organizations (NGOs). Minimum standards for patent law exist internationally, as evidenced by Article 27 of the Trade-Related Aspects of Intellectual Property Rights (TRIPs) agreement. This article provides that “patents shall be available for any inventions, whether products or processes, in all fields of technology, provided that they are new, involve an incentive step and are capable of industrial application. However, nations can choose to exclude certain inventions, such as those that harm the public, types of medical treatments, and certain plants from patentability. Individual nations have the power to interpret the terms of such agreements in terms as broad or narrow as they wish. Agreements such as TRIPs have been criticized as instruments used to secure enforcement of US intellectual property rights abroad [6]. In the words of well known Indian environmental activist Vandana Shiva, “Patents are given for an invention and a patent on life necessarily means biopiracy.” If a patent does not respect one of the three criteria for access (novelty, inventiveness and commercial application), it is not legally admissible and must be canceled. Thus, a patent based on traditional knowledge is illegal, because it does not respect the principle of novelty, nor does it respect the criteria of inventiveness [3]. INTERNATIONAL TREATIES DEALING WITH BIOPIRACY There are two international treaties that help protect peoples and biodiversity from biopirates: 1. Convention on Biological Diversity (CBD) evolved of the Rio Earth Summit (1992). 2. Nagoya Protocol on biodiversity, negotiated in Japan (2010). Before these two treaties, one earlier attempt for the protection of indigenous knowledge (IK) or TK was made in 1994 through the United Nations Draft Declaration on the Rights of Indigenous People. There is a clear provision in the Declaration to recognize the ownership of the IK of the indigenous communities. Article 29 mandates the recognition of the full ownership, control and protection of their cultural and intellectual property. This includes human and other genetic resources, seeds, medicines, knowledge of the properties of fauna and flora, oral traditions, literature, designs and visual and performing arts. They are also given rights to manage them [2]. The International Labour Organization (ILO) also emphasized the rights of indigenous people and the need for their recognition. The Indigenous and Tribal Peoples Convention (Convention 169), 1989, recognized the aspirations of these peoples to exercise control over their own institutions, ways of life and economic development, and to maintain and develop their identities, languages and religions within the framework of the States in which they live. The Convention states that the rights of these peoples related to the natural resources pertaining to their lands shall be specifically safeguarded. These rights include that of participation in the use, management and conservation of these resources [Article 15(1)] [2]. CONVENTION ON BIOLOGICAL DIVERSITY (CBD): In 1992, the international community designed the Convention on Biological Diversity (CBD) as a new instrument to prevent the loss of biodiversity worldwide. Realizing the importance of indigenous knowledge (IK) and the objective of the CBD with regard to benefit-sharing, the Conference of Parties to the CBD, in its sixth meeting (COP 6) in 2002, adopted the Bonn Guidelines on Access to Genetic Resources and Fair and Equitable Sharing of the Benefits Arising out of their Utilization. It provides the guidelines for developing legislative, administrative or policy measures on access and benefit-sharing. Articles 15 and 8 (j) of this convention are important and define the legal principles and criteria for bio-trade, involving the use of natural native ingredients, and often traditional knowledge concerned. CBD sets up three complementary objectives i.e. the conservation of biodiversity, its sustainable use, and the fair and equitable sharing of the benefit arising from their use which is also known as the “access and benefit sharing (ABS)” mechanism. The CBD recognizes the value of the ‘knowledge, innovations and practices of indigenous and local communities’ for the conservation and sustainable use of biological diversity. The main issue is that of Access and Benefit Sharing (ABS). ABS is implemented under Article 15 of CBD under which national governments are required to establish domestic laws and policies to allow access to genetic resources. It aims to eradicate the evils of biopiracy as well as protect the interests of TK holders [2, 3, 9]. NAGOYA PROTOCOL The Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits is a landmark treaty that was devised keeping in mind the increasing loss of biodiversity on Earth. The Nagoya Protocol specifies the means by which the CBD can be applied. The Nagoya Protocol, especially the ABS clause, calls for systems to be put in place [9].These systems are expected to drive the costs incurred by pharmaceutical companies during the drug discovery phase. The Nagoya Protocol could have an adverse impact on the pharmaceutical industry. Many companies feel that the Access and Benefit Sharing clause will increase product development costs and complicate the drug discovery phase. According to the protocol, organizations will have to pay a significant amount of their revenue and royalties to indigenous communities and host countries for the drug they develop from genetic resources. The revised patent system will also add to the cost of drug development. Due to the rules and regulations laid down by the Nagoya Protocol, organizations would have to execute joint patents with the communities from whom they source resources [7]. INDIA FIGHTS AGAINST BIOPIRACY India is one of the 17 mega-biodiversity countries with 2.4 per cent of the global land area and accounts for 7 to 8 per cent of the recorded species of the world, making it more prone to biopiracy [10]. The turmeric case, in which India succeeded in overturning a patent granted by the United States Patent and Trademark Office on turmeric powder, was a landmark in the battle against ‘bio-piracy’. It was the first case in which a Third World country succeeded in its objection to a foreign patent on the grounds that it was based on traditional knowledge known to the country for generations. In 1995, two expatriate Indians at the University of Mississippi Medical Centre (Suman K. Das and Harihar P. Cohly) were granted a US patent (no. 5,401,504) on use of turmeric in wound healing. The Council of Scientific & Industrial Research (CSIR), India, New Delhi filed a re-examination case with the US PTO challenging the patent on the grounds of existing of prior art. CSIR argued that turmeric has been used for thousands of years for healing wounds and rashes and therefore its medicinal use was not a novel invention. Their claim was supported by documentary evidence of traditional knowledge, including ancient Sanskrit text and a paper published in 1953 in the Journal of the Indian Medical Association. Despite an appeal by the patent holders, the US PTO upheld the CSIR objections and cancelled the patent. Grant of patent for fungicidal effect of neem oil in 1994 is another such example. European Patent Office (EPO) granted a patent (No.436257) to the US Corporation W.R. Grace Company and US Department of Agriculture for a method for controlling fungi on plants by the aid of hydrophobic extracted Neem oil. In 1995, a group of international NGOs and representatives of Indian farmers filed legal opposition against the patent. They submitted evidence that the fungicidal effect of extracts of Neem seeds had been known and used for centuries in Indian agriculture to protect crops, and therefore, cannot be patented. In 1999, the EPO determined that according to the evidence all features of the present claim were disclosed to the public prior to the patent application and the patent was not considered to involve an inventive step. The patent granted on was Neem was revoked by the EPO in May 2000 [11, 12]. Learning from such bitter experiences, Indian government framed following acts and projects to fight against biopiracy:1. BIOLOGICAL DIVERSITY ACT (2002), INDIA In India, enabling provisions have been made for protecting the traditional knowledge in the Biodiversity Bill 2000. Section 36(iv) provides for protection of knowledge of local people relating to biodiversity through measures such as registration of such knowledge, and development of a sui generis system. For ensuring equitable sharing of benefits arising from the use of biological resources and associated knowledge, sections 19 and 21 stipulate prior approval of the National Biodiversity Authority (NBA) before their access. Section 6 provides that anybody seeking any kind of intellectual property rights on a research based upon biological resource or knowledge obtained from India; need to obtain prior approval of the NBA. The NBA will impose benefit-sharing conditions. Section 18(iv) stipulates that one of the functions of NBA is to take measures to oppose the grant of IPRs in any country outside India on any biological resource obtained from India or knowledge associated with such biological resource. India's Biological Diversity Act, 2002 (BDA) seeks to do several tasks, including to regulate access to biological resources with the purpose of securing equitable share in benefits arising out of the use of biological resources and associated TK to conserve and sustainably use biological diversity; to respect and protect TK of local communities to secure sharing of benefits with local people as conservers of biological resources and TK holders. The Biological Diversity Rules of 2004 further identifies benefit sharing methods such as joint ventures, technology transfer, product development, education, awareness raising, institutional capacity building, and venture capital funds, and states that applications will be determined on a case by case basis [10, 13]. 2. PROTECTION OF PLANT VARIETIES (PPV) AND FARMERS’ RIGHTS ACT (2001) The Indian legislation for the Protection of Plant Varieties and Farmers’ Rights Act, 2001, also acknowledge that the conservation, exploration, collection, characterization, evaluation of plant genetic resources for food and agriculture are essential to meet the goals of national food and nutritional security as also for sustainable development of agriculture for the present and future generations [5]. 3. TRADITIONAL KNOWLEDGE DIGITAL LIBRARY (TKDL) India has taken various initiatives regarding the protection of traditional knowledge under intellectual property rights, including the Traditional Knowledge Digital Library (TKDL), to protect its traditional knowledge and to prevent grant of wrong patents. A collaborative project between CSIR and Department of AYUSH, Ministry of Health and Family Welfare, TKDL is a maiden Indian effort to help prevent misappropriation of traditional knowledge belonging to India at International Patent Offices. By recording the traditional knowledge, legally, it becomes public domain knowledge. Under the patent law, this means that it is considered to be prior art and hence is not patentable. Such a written record, in a form easily accessible to patent offices around the world, would provide all such offices with a record of India’s prior art. Patent examiners could easily check this database and reject any patent application that might be a mere copy of traditional knowledge. Being in document form, it would be acceptable to patent offices that insist on a written record of prior art, as in the United States. To this extent it would prevent cases of ‘biopiracy’. Around the time the TKDL was established in 2001, the TKDL expert group estimated that, annually, some 2,000 patents relating to Indian medicinal systems were being erroneously granted by patent offices around the world. TKDL thus enables cancellation/withdrawal of wrong patent applications concerning India’s traditional knowledge at zero cost and in few weeks time [12, 14]. CONCLUSIONS Biopiracy is immerging scientific nuisance in pharmaceutical business. It can commercialize locally as well as globally well known facts, inherited knowledge, traditional knowledge, community wisdom, etc., in order to explore new opportunity and cost saving in pharmaceuticals research and development. CBD and NAGOYA protocol have tried to optimize the conflict between bio-pirates and original resources bearers by proposing regulatory understanding which still needs to be revised and redefined. In India, NBDA and TKDL are two start-up initiatives to counter act the biopiracy. REFERENCES 1. WIPO. (2013) Traditional Knowledge, Available online: http://www.wipo.int/tk/en/tk/ (updated: August 2013). 2. Sahay S, Pavithran P, Barpujari I: “Biopiracy: Imitations Not Innovations”, Gene Campaign Publication, New Delhi, 2007. 3. Understanding, resisting and acting against biopiracy. Available online at http://www.biopiraterie.org/sites/default/files/etudes/Livret_Uk_010612.pdf (accessed September, 2013) The (French) Biopiracy Collective. 4. Stiglitz JE: “Globalization and its discontents”, W.W. Norton & Company, New York, 2002. 5. Chouhan VK. (2012) Protection of Traditional Knowledge in India by Patent: Legal Aspect. IOSR Journal of Humanities and Social Science. 3(1): 35-42. 6. Mgbeoji I: “Global Biopiracy: Patents, Plants and Indigenous Knowledge”, Cornell University Press, Ithaca, 2006. Pp. 312. 7. Kurien K, Das A. (2011) Nagoya Protocol and Its Implications on Pharmaceutical Industry. Beroe Inc. Publication, pp. 3-6. 8. Zakrzewski PA. (2002) Bioprospecting or Biopiracy? The Pharmaceutical Industry’s Use of Indigenous Medicinal Plants as a Source of Potential Drug Candidates. Compl. Alternat. Med. 79(3): 252-254 9. http://www.cbd.int/convention/text 10. http://www.nbaindia.org 11. ENVIS Newsletter. (2011) Envis Centre on Biotechnology, Vol. 19, Department of Environmental Science, University of Kalyani, Nadia, West Bengal. 12. Udgaonkar S. (2002) The recording of traditional knowledge: Will it prevent ‘bio-piracy’? Current Sci. 82(4): 413-419. 13. India and the WTO newsletter. (2004) Ministry of Commerce & Industry, Government of India publication, Udyog Bhawan, New Delhi, 4(4): 7-19. 14. Gupta VK. (2011) Protecting India’s Traditional Knowledge. WIPO Magazine, Available online: http://www.wipo.int/wipo_magazine/en/2011/03/ article_0002.html (updated: June 2011).