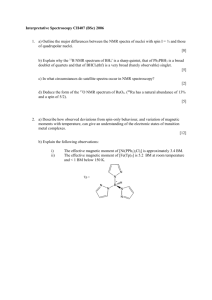
Biotechnology Example B

Protein folding and Unfolding Using Florescent Proteins
The experiment you are about to do was inspired by an experiment performed by Kurt Wuthrich around 1967. Dr. Wuthrich shared the 2002
Nobel Prize in Chemistry "for his development of nuclear magnetic resonance spectroscopy for determining the three-dimensional structure of biological macromolecules in solution" (1). One major difference is that C-
13 NMR was not as convenient and available then as now. The basis of the experiment is that the magnetic environments of the protons or C-13 atoms attached to the residues of a folded protein will have a different chemical shift for those in an unfolded protein.
A brief NMR Primer
NMR translates to Nuclear Magnetic Resonance
Nuclear refers to the particle which is absorbing radio frequency energy.
(This absorption causes a change in spin states, see below)
Magnetic –A magnet is used to make the normally degenerate (same energy) spin states observable.
Resonance – When the radio frequency (Rf) of the instrument matches the natural frequency of the nuclei, the nuclei spin flip or change spin.
Spin flips are recorded as energy absorptions.
Copyright 2010 © C. Frederick Jury, Ph.D. Collin College 1
Here are two Methanol spectra. TMS (TetraMethylSilane)(at the right on spectrum above) is an internal standard used to set the instrument scale.
TMS is always located at zero ppm.
Now for the terms shielded and desheilded or Upfield and Downfield.
Nuclei which are surrounded by electrons will have a hard time “interacting” with the applied field of the instrument , hence they are said to be shielded.
Nuclei which are bonded near groups with higher electronegativity relative to carbon (electron withdrawing groups) will be “De-shielded” because the electron density around these nuclei will be reduced.
Copyright 2010 © C. Frederick Jury, Ph.D. Collin College 2
Chemical shift refers to the distance between two resonances (or peaks).
This parameter gives an indication of the micro magnetic environment in which that particular nucleus is located.
The experiment:
This experiment uses EMGFP, However any fluorescent protein could be used.
1. GFP; 2.0 ml an stock aqueous solution of Green Fluorescent Protein
(EMGFP) will be added to an NMR tube and the proton NMR spectrum will be recorded (Spectrum #1). The C-13 spectrum will also be recorded.
(Spectrum #1A). The protein will be fluorescent when folded properly and will be observable.
2. The stock solution will then be transferred to a new tube and the pH adjusted up or down until the EMGFP no longer fluoresces. Proton and C-13
NMR spectra will then be recorded (Spectra #2 & #2A). If a precipitate forms you must repeat the pH adjustment, stopping prior to precipation.
If a precipitate forms , Does this give any information about the structure of the protein?
Analyze the spectra by comparing the chemical shifts of the major peaks shown in spectra #1 vs #2 and #1A vs #2A .
Assuming the original spectrum is that of a folded protein, what conclusions can be drawn about the structure of the protein in spectra #2 & 2A?
How do you know if the original protein is folded properly?
Another NMR feature that can be correlated to structure is the J value or coupling constant for a set of magnetically interacting nuclei. This parameter can be associated with the distance separating the interacting nuclei. A 2D
COSY spectrum can be performed on both samples if time allows to look for changes in J values.
1. http://nobelprize.org/nobel_prizes/chemistry/laureates/2002/
Copyright 2010 © C. Frederick Jury, Ph.D. Collin College 3