exam guidance for revision
advertisement
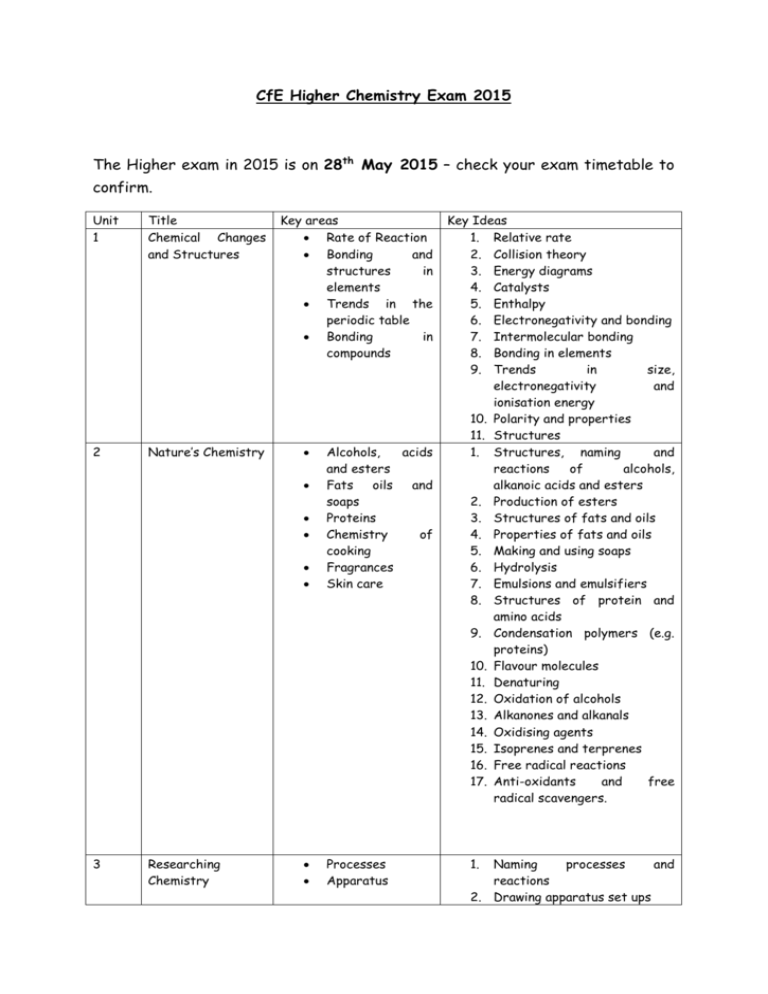
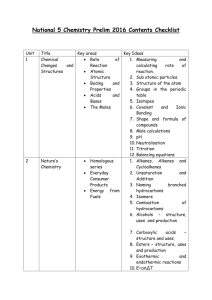
CfE Higher Chemistry Exam 2015 The Higher exam in 2015 is on 28th May 2015 – check your exam timetable to confirm. Unit 1 Title Chemical Changes and Structures 2 Nature’s Chemistry Key areas Rate of Reaction Bonding and structures in elements Trends in the periodic table Bonding in compounds Alcohols, acids and esters Fats oils and soaps Proteins Chemistry of cooking Fragrances Skin care Processes Apparatus 3 Researching Chemistry Key Ideas 1. Relative rate 2. Collision theory 3. Energy diagrams 4. Catalysts 5. Enthalpy 6. Electronegativity and bonding 7. Intermolecular bonding 8. Bonding in elements 9. Trends in size, electronegativity and ionisation energy 10. Polarity and properties 11. Structures 1. Structures, naming and reactions of alcohols, alkanoic acids and esters 2. Production of esters 3. Structures of fats and oils 4. Properties of fats and oils 5. Making and using soaps 6. Hydrolysis 7. Emulsions and emulsifiers 8. Structures of protein and amino acids 9. Condensation polymers (e.g. proteins) 10. Flavour molecules 11. Denaturing 12. Oxidation of alcohols 13. Alkanones and alkanals 14. Oxidising agents 15. Isoprenes and terprenes 16. Free radical reactions 17. Anti-oxidants and free radical scavengers. 1. Naming processes and reactions 2. Drawing apparatus set ups 4 Chemistry Society in Industrial Processes Mole calculations Equilibria Chemical Energy Redox Chromatography Volumetric analysis 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. Costs, raw materials etc. and processes Calculations from equations Molar volume Excess Percentage yield Atom economy Equilibrium Le Chatelier’s principle Enthalpies of combustion, solution and neutralisation Hess’ Law Ion-electron equations – balancing and combining Oxidation and reduction Oxidising and reducing agents TLC/ paper chromatography GLC/ column chromatography Rf values Calculations using information from titration If you do not understand something – please do not spend hours wasting revision time. Please come into school and ask – you will be welcomed at any time (although you may have to wait for an hour until we finish teaching a class). You could also email into school to ask your teacher a question (during the holidays teachers cannot use their school email addresses. Sources of Revision Material: Revision Notes on School website (Chemistry>Courses>Higher>Resources) Course booklets Websites – BBC bitesize and evans2chemweb (username Kinross and password Pringle). The scholar website also gives useful help. (Make sure you know your unique password –available from your teacher) The SQA publishes past papers and a list of questions from the previous Higher that are suitable can be found at: http://www.sqa.org.uk/sqa/47913.html A limited number of commercial materials are also available to borrow from the department.