BME 272 NCIIA Grant Proposal Design of a MK2i
advertisement

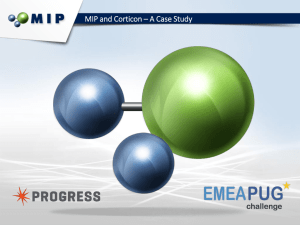
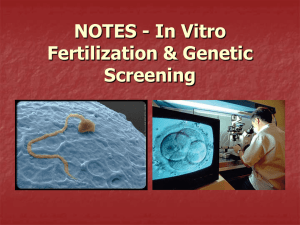
BME 272 NCIIA Grant Proposal Design of a MK2i-loaded PLLA Nanofilm to Combat Intimal Hyperplasia in Autologous Venous Grafts Mitchell Weisenberger, James Carrow, Ming Chen, Andrew Schultze Team GraftWrap December 6, 2012 Abstract Intimal hyperplasia, a major cause of autologous graft failure in coronary artery bypass graft (CABG) surgery, is mediated by the activation of intracellular kinase Mitogen Activated Protein Kinase Activated Protein Kinase 2 (MK2). MK2 activation leads to smooth muscle cell (SMC) infiltration into the vessel lumen and subsequent graft occlusion. A cell penetrating peptide (CPP) conjugated inhibitory sequence (YARAMK2i) has been shown to block MK2 and reduce downstream inflammatory markers in vitro, yet an adequate delivery method for this therapeutic to graft SMCs during and following CABG surgery is an unmet challenge. Separately, PLLA nanofilms have been developed which can load drugs, adhere to wet tissues, and degrade slowly in vitro. We wish to develop a YARAMK2i-loaded PLLA nanofilm for direct application to the CABG outer surface to serve as a drug depot for extended delivery of YARAMK2i and deterrence of intimal hyperplasia. Our primary objectives are to: Synthesize YARAMK2i-loaded PLLA nanofilms of various thicknesses and confirm film thicknesses via atomic force microscopy (AFM). Conduct drug release and degradation assays to determine release kinetics and degradation properties of loaded nanofilms respectively, followed by determination of optimal nanofilm thickness for drug delivery. Undertake cytotoxicity and ELISA assays to determine biocompatibility and therapeutic potential of loaded nanofilms in vitro, followed by determination of optimal drug loading concentration. Introduction Coronary Heart Disease and Intimal Hyperplasia: A primary health concern that has emerged due to recent lifestyle developments is the occlusion of the blood vessels which nourish the heart, leading to ischemia and mechanical failure of cardiac tissue. We know it is the leading cause of death in America, and in 2008 its effects resulted in one sixth of the total deaths in the U.S. (1). As of now, standard treatment for a blocked coronary artery is an autograph from the saphenous vein, which is used to bypass the blockage and return blood flow. Yet, Figure 1: Intimal hyperplasia (right) in almost 20% of these grafts fail in the first year alone (2) comparison to healthy venous tissue (left) and thus there is recognizable room for improvement in entails SMC migration and ECM deposition the therapy. into the lumen without distinct organization. The failure is attributed to the transformation of vascular smooth muscle cells (VSMCs) in the graft into a pathological state, which causes them to grow into the lumen of the blood vessel. This leads to the narrowing and even blockage of the graft itself, a process termed intimal hyperplasia (Fig. 1) (3). Under further investigation, we can dissect the pathway in which the vessels undergo this physiological change. Within this pathway, MK2 is an intracellular kinase which phosphorylates heat shock protein 27. From this, actin dynamics are altered and result in SMC proliferation and migration into the vessel lumen (Fig. 2). This will be our target of the treatment. During coronary artery bypass surgery, activation of MK2 leads to smooth muscle cell infiltration into the vessel lumen and subsequent graft occlusion. Currently MK2i has been used to inhibit the activation of MK2, reducing the amount of smooth muscle cell infiltration, which in turn reduces occlusion within the vessel graft. The problem which we look to address is the short timeframe in which the graft is treated with MK2i. We will do this by integrating MK2i into a nanosheet, which when wrapped around the graft, will release MK2i over a longer period of time. This would reduce the activation of MK2 for a longer time than is currently in practice. Nanofilms for Drug Delivery: By encapsulating the YARAMK2i in a PLLA nanofilm, we will have more control of the release mechanism and limit immediate release of the peptide. We hope that the release profile will resemble Fickian diffusion rather than straight dissolution. By tuning our thickness of the film and drug integration method, we hope to delay the release of the YARAMK2i for Figure 2: The molecular mechanism of VSMC pathogenesis is specifically mediated by maximal cellular response. Considerations of nanofilm MK2. adhesivity will also dictate thickness and loading method. As coronary artery bypass surgery is becoming more prevalent in the world, it is necessary to increase the effectiveness of the surgery. As 20% of grafts fail within the first year due to intimal hyperplasia, there is a profound need to find a way to combat occlusion of the vessel graft. The YARAMK2i nanosheet we will develop will decrease the incidence of intimal hyperplasia after coronary artery bypass surgery, and decrease the likelihood that the graft will fail. History YARAMK2i: • An inhibitory sequence (MK2i) has been developed which binds MK2 and deters the process of intimal hyperplasia (4), yet an adequate delivery technology is needed. • The CPP-conjugated MK2i (YARAMK2i) has been shown to exhibit MK2 and downstream inflammatory marker inhibition in vitro (5). • This YARAMK2i therapeutic has been successfully synthesized and isolated before by Mitchell Weisenberger (BME) in Vanderbilt’s Advanced Therapeutic Laboratory directed by Professor Craig Duvall. • Professor Craig Duvall has granted his permission to utilize his utilities to synthesize, characterize, and isolate the YARAMK2i therapeutic for the project. PLLA Nanofilms: • • • • • • • PLLA is an FDA approved material for medical applications. PLLA nanofilms less than 100 nm thick were seen to be stable for more than 3 weeks in vitro in conditions of a pH of 1 and .5% pepsin, and the sheets also served to facilitate closing of a gastric lesion in vivo after 7 days (6). This suggests that PLLA nanofilms could serve as a biomaterial that would slowly degrade over time when wrapped around a CABG. PLLA nanofilms less than 100 nm thick were observed to adhere strongly to wet tissue and organs (6), suggesting that they could adhere well to the outside of a CAGB. Superparamagnetic iron oxide nanoparticle loaded PLLA nanosheets have been successfully manufactured with a homogenous distribution of nanoparticles (7), suggesting the feasibility of YARAMK2i-loaded nanosheets. PLLA nanosheets of various thicknesses and layers have been synthesized by Jake Carrow (BME) in Phil Messersmith’s lab in Northwestern’s BME department. At Northwestern, films were synthesized to carry a wound healing drug with release profiles measured Currently at Vanderbilt, nanosheets have been loaded with gold nanoparticles for a laser stimulation project, but none have been loaded with YARAMK2i presently Team Undergraduates: 1. Mitchell Weisenberger (BME): Has synthesized YARAMK2i before in Doctor Craig Duvall’s lab, and has experience in solid phase peptide synthesis, isolation via high performance liquid chromatography (HPLC), and identification via liquid chromatography mass spectroscopy (LCMS). Has worked with cells. Will be especially focused on the synthesis of YARAMK2i and in vitro analysis of loaded films. 2. James (Jake) Carrow (BME): Has synthesized multi-layer PLLA nanofilms via spin coating techniques in Phil Messersmith’s Northwestern University BME lab, and will be working with such films this year in Doctor Giorgio’s lab. Is proficient in organic chemistry. Has worked with cells. Will be especially focused on the loading and synthesis of the PLLA nanofilms and in vitro analysis of loaded films. 3. Ming Cheng (BME): Has worked on peptide synthesis, HPLC, and polymer chemistry in Doctor Duvall’s lab. Has worked with cells. Will be especially focused on the synthesis of YARAMK2i and in vitro analysis of loaded films. 4. Andrew Schultze (BME): Has worked with dynamic mechanical testing techniques in Doctor Nyman’s lab. Will be especially focused on the mechanical application of films to cell cultures during in vitro analysis, as well as mechanical characterization of films should the interest arise. Directors: 1. Doctor Craig Duvall 2. Doctor Hak-Joon Sung 3. Doctor Todd Giorgio Work Plans and Outcomes 10/1/2012 11/1/2012 12/1/2012 1/1/2013 2/1/2013 3/3/2013 Synthesis of YARAMK2i YARAMK2i purification and conjugation of FITC Creation of YARAMK2i-FITC-loaded PLLA nanofilms Start Date Characterization of nanofilm thicknesses Completed Drug release and nanofilm degredation assays Remaining Determination of optimal nanofilm thickness In vitro culture of loaded films and biological assays Determination of optimal nanofilm drug loading concentration Major Outcome: We wish to ultimately construct a YARAMK2i-loaded nanofilm of optimal thickness and loading concentration which is biocompatible and can cause inflammatory marker knockdown in vitro. A loaded nanofilm of optimal thickness will be able to adhere to wet tissue in vitro while still being sufficiently thick enough to display release kinetics that elicit an optimal therapeutic effect in in vitro ELISA assays. Optimal loading concentration will be achieved when the loaded film elicits a peak therapeutic effect in in vitro ELISA inflammatory marker knockdown without causing a significantly greater cytotoxicity in vitro in comparison to no treatment. Inflammatory marker knockdown in vitro will be dictated by ELISA assays which display a significant decrease in inflammatory markers, either IL-6 or TNF-alpha, in comparison to no treatment in an in vitro cell culture of endothelial cells. The PLLA-loaded nanosheets fit into the lab culture of the Duvall, Sung, and Giorgio labs in that they are drug delivery platforms that may be loaded with other compounds for therapeutic treatments. Sources (1) Roger, V. L., A. S. Go, et al. (2011). "Heart Disease and Stroke Statistics—2012 Update." Circulation. (2) Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996;28:616–626 (3) Glagov, S. (1994). "Intimal hyperplasia, vascular modeling, and the restenosis problem." Circulation 89(6): 2888-2891. (4) Hayess, K. and R. Benndorf (1997). "Effect of protein kinase inhibitors on activity of mammalian small heat-shock protein (HSP25) kinase." Biochemical Pharmacology 53(9): 1239-1247. (5) Brugnano, J. L., B. K. Chan, et al. (2011). "Cell-penetrating peptides can confer biological function: Regulation of inflammatory cytokines in human monocytes by MK2 inhibitor peptides." Journal of Controlled Release 155(2): 128-133. (7) Okamura, Y., Kabata, K., Kinoshita, M., Saitoh, D. and Takeoka, S. (2009), FreeStanding Biodegradable Poly(lactic acid) Nanosheet for Sealing Operations in Surgery. Adv. Mater., 21: 4388–4392. doi: 10.1002/adma.200901035