Exempt Dealing application form
advertisement

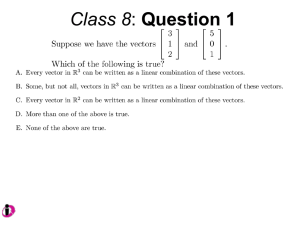
University of Sydney Institutional Biosafety Committee This form is to be completed by the Principal Investigator and must be submitted by the Principal Investigator Name of Principal Investigator: Project title: Type of project proposal: □ Exempt Dealing □ NLRD □ DNIR RIMS Project ID Code: (for projects that are part of a funded research grant ) For completion by IBC. IBC Reference Number Name of IBC Chair Prof Anthony Weiss Signature Exempt Dealing application form – May 2015 Date of approval Page 1 University of Sydney Institutional Biosafety Committee – Exempt Dealing application form 1. Exemption Category – Please place an X in the appropriate box/s OGTR Mark Item # with an X 2 Description of Dealing A dealing with a genetically modified Caenorhabditis elegans, whereby: a) an advantage is not conferred on the animal by the genetic modification; and b) as a result of the genetic modification, the animal is not capable of secreting or producing an infectious agent. 3 A dealing with an animal into which genetically modified somatic cells have been introduced, if: a) the somatic cells are not capable of giving rise to infectious agents as a result of the genetic modification; and b) the animal is not infected with a virus that is capable of recombining with the genetically modified nucleic acid in the somatic cells. 3A A dealing with an animal whose somatic cells have been genetically modified in vivo by a replication defective viral vector, if: a) the in vivo modification occurred as part of a previous dealing; and b) the replication defective viral vector is no longer in the animal; and c) no germ line cells have been genetically modified; and d) the somatic cells cannot give rise to infectious agents as a result of the genetic modification; and e) the animal is not infected with a virus that can recombine with the genetically modified nucleic acid in the somatic cells of the animal. Exempt Dealing application form – May 2015 Page 2 4 1) Subject to subitem 2) below, a dealing involving a host/vector system mentioned in Part 2 of Schedule 2 and producing no more than 25 litres of GMO culture in each vessel containing the resultant culture. 2) The donor nucleic acid: a) must meet either of the following requirements: i) it must not be derived from organisms implicated in, or with a history of causing, disease in otherwise healthy human beings, animal, plants or fungi; ii) it must be characterised and the information derived from its characterisation show that it is unlikely to increase the capacity of the host or vector to cause harm; and b) must not code for a toxin with an LD50 of less than 100µg/kg; and c) must not code for a toxin with an LD50 of 100µg/kg or more, if the intention is to express the toxin at high levels; and d) must not be uncharacterised nucleic acid from a toxin-producing organism; and e) must not include a viral sequence, unless the donor nucleic acid: i) is missing at least 1 gene essential for viral multiplication that: A) is not available in the cell into which the nucleic acid is introduced; and B) will not become available during the dealing; and ii) cannot restore replication competence to the vector. 5 A dealing involving shot-gun cloning, or the preparation of a cDNA library, in a host/vector system mentioned in item 1 of Part 2 of Schedule 2, if the donor nucleic acid is not derived from either: a) a pathogen; or b) a toxin-producing organism. 2. Name and full professional address of Principal Investigator submitting proposal Name: Department: Room No: Building Name: Tel: Email: 3. Name(s) of other Principal Investigators responsible for the project. Please provide their professional addresses if different from that above. Exempt Dealing application form – May 2015 Page 3 4. Describe the aim of the work 5. Describe the main experimental procedures of the work 6. Details of biological system Note: Any substantial change in the system will require submission of another proposal (a) Describe the biological source of the donor DNA to be used – include the genus, species and strain or organ/tissue as applicable. Include the specific genes to be involved in the dealing. (b) Describe the host organism or tissue to be used – include the genus, species and strain where applicable. If not a commonly used laboratory strain, include the name of the strain from which it is derived. (c) Describe the vectors or methods to be used to transfer donor DNA to the host. Include information regarding the origin and properties of the vector and confirm that all bacterial plasmid vectors are non-conjugative. If retroviral vectors are to be used, include the viral envelope to be used. (d) If your project uses GMOs created by replication defective viral vectors from a previous dealing, select all system(s) that were used: Retroviral/Lentiviral vectors unable to transduce human cells – Complete Q7 Retroviral/Lentiviral vectors that can transduce human cells – Complete Q8 Adenoviral/Adeno-associated viral vectors – Complete Q9 Exempt Dealing application form – May 2015 Page 4 7. Retroviral/Lentiviral vectors unable to transduce human cells (a) Please give a name, reference (literature or website) and brief description of the viral vector that was used to genetically modify the cell line or animal (b) Please provide either: i) the USyd IBC reference number for the previous NLRD dealing and viral envelope used. OR ii) details of the company/collaborator providing the modified cell line or animal transduced with replication defective viral vector, and the viral envelope used. 8. Retroviral/Lentiviral vectors that can transduce human cells (a) Please give a name, reference (literature or website) and brief description of the viral vector that was used to genetically modify the cell line or animal (b) Please provide either: i) the USyd IBC reference number for the previous NLRD dealing and viral envelope used. OR ii) details of the company/collaborator providing the modified cell line or animal transduced with replication defective viral vector, and the viral envelope used. (c) Is the viral vector that was used a third generation* replication defective retroviral/lentiviral vector? Yes - proceed to 8(d) No – Please provide an explicit description of the assay that was performed to exclude the presence of replication competent virus. Please provide a copy of the reference for your intended assay. Exempt Dealing application form – May 2015 Page 5 * N.B. 3rd generation packaging systems fulfill the following criteria: (i) all viral genes have been removed from the retroviral vector so that it cannot replicate or assemble into a virion without these functions being supplied in trans: and (ii) viral genes needed for virion production in the packaging cell line are expressed from independent, unlinked loci with minimal sequence overlap with the vector to limit or prevent recombination; and (iii) either: (A) the retroviral vector includes a deletion in the Long Terminal Repeat sequence of DNA that prevents transcription of genomic RNA following integration into the host cell DNA; or (B) the packaging cell line and packaging plasmids express only viral genes gagpol, rev and an envelope protein gene, or subset of these (d) Have cell cultures been washed ≥2 times 4-6 hours after transduction with 3rd generation lentiviral vectors and then incubated for ≥72h? Yes N/A (e) For laboratory animals, has at least 3 days elapsed between exposure to the 3rd generation lentiviral vectors and the proposed exempt dealing? Yes N/A Exempt Dealing application form – May 2015 Page 6 9. Adenoviral/Adeno-Associated viral vectors (a) Please provide either: i) the USyd IBC reference number for the previous NLRD dealing. ii) details of the company/collaborator providing the modified cell line or animal transduced with AAV or Adenoviral vector. (b) Please give a name, reference (literature or website) and brief description of the viral vector that was used to genetically modify the cell line or animal, including the strain of virus and biosafety characteristics (e.g. whether or not helper virus was used to make it). (c) For a cell line, please provide details of how many washes/passages have been undertaken since the initial dealing (d) For a dealing with an animal, please provide details of how much time will have passed between the exposure of the animal to the vector and the proposed dealing. (e) Please provide evidence of the half-life of each AAV or adenovirus used in your chosen cell lines or animal models to justify why you believe no vector particles remain. Exempt Dealing application form – May 2015 Page 7 10. If you believe the protein/gene is characterised, and unlikely to increase the capacity of the host or vector to cause harm, briefly explain why, referring to what is known about its structure, function and/or genetics. 11. Does the DNA encode a toxin? □ Yes □ No If yes, please provide the LD50 12. Where will this work be conducted? Please provide Room Number, Building Name and Building Code. 13. Do you have approval to use this facility? Yes [ ] No [ ] 14. Proposed date of commencement of work. 15. Likely duration of work. 16. Signature of Principal Investigator submitting this proposal. Date Exempt Dealing application form – May 2015 / / Page 8