New York State Medicaid Preferred Drug Program
Opioid Agents Prior Authorization Worksheet
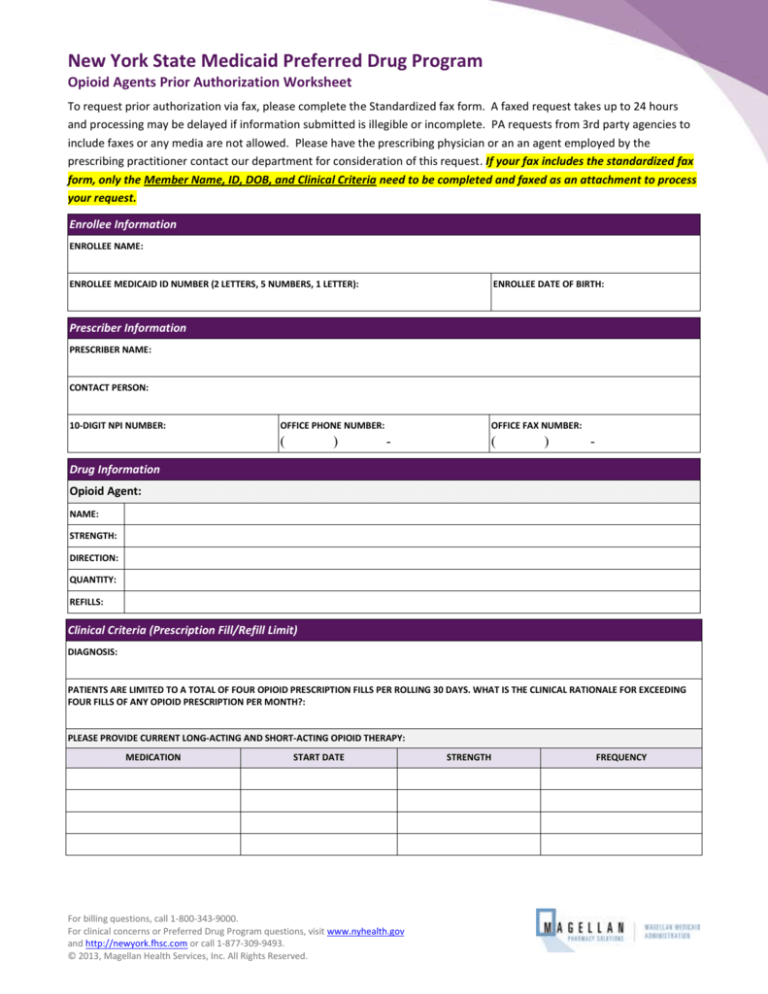
To request prior authorization via fax, please complete the Standardized fax form. A faxed request takes up to 24 hours
and processing may be delayed if information submitted is illegible or incomplete. PA requests from 3rd party agencies to
include faxes or any media are not allowed. Please have the prescribing physician or an an agent employed by the
prescribing practitioner contact our department for consideration of this request. If your fax includes the standardized fax
form, only the Member Name, ID, DOB, and Clinical Criteria need to be completed and faxed as an attachment to process
your request.
Enrollee Information
ENROLLEE NAME:
ENROLLEE MEDICAID ID NUMBER (2 LETTERS, 5 NUMBERS, 1 LETTER):
ENROLLEE DATE OF BIRTH:
Prescriber Information
PRESCRIBER NAME:
CONTACT PERSON:
10-DIGIT NPI NUMBER:
OFFICE PHONE NUMBER:
(
)
OFFICE FAX NUMBER:
-
(
)
-
Drug Information
Opioid Agent:
NAME:
STRENGTH:
DIRECTION:
QUANTITY:
REFILLS:
Clinical Criteria (Prescription Fill/Refill Limit)
DIAGNOSIS:
PATIENTS ARE LIMITED TO A TOTAL OF FOUR OPIOID PRESCRIPTION FILLS PER ROLLING 30 DAYS. WHAT IS THE CLINICAL RATIONALE FOR EXCEEDING
FOUR FILLS OF ANY OPIOID PRESCRIPTION PER MONTH?:
PLEASE PROVIDE CURRENT LONG-ACTING AND SHORT-ACTING OPIOID THERAPY:
MEDICATION
START DATE
For billing questions, call 1-800-343-9000.
For clinical concerns or Preferred Drug Program questions, visit www.nyhealth.gov
and http://newyork.fhsc.com or call 1-877-309-9493.
© 2013, Magellan Health Services, Inc. All Rights Reserved.
STRENGTH
FREQUENCY
Magellan Medicaid Administration
Opioid Agents Prior Authorization Fax Form
Preferred Drug List
IS A NON-PREFERRED OPIOID AGENT BEING PRESCRIBED? (PLEASE REFER TO THE PDL AT NEWYORK.FHSC.COM FOR A CURRENT LIST OF PREFERRED
AND NON-PREFERRED AGENTS)
Yes
No
IF YES, PLEASE PROVIDE CLINICAL RATIONALE FOR USE OF A NON-PREFERRED AGENT (FORM CANNOT BE PROCESSED WITHOUT REQUIRED
EXPLANATION):
Patient has experienced a treatment failure with a preferred drug.
Yes
No
Patient has experienced an adverse drug reaction with a preferred drug.
Yes
No
There is a documented history of successful therapeutic control with a nonpreferred drug and transition to a
preferred drug is medically contraindicated.
Yes
No
Other (Please specify the clinical reason the patient is unable to use a preferred agent in the same drug class. If necessary, fax
additional pages):
Step Therapy
Nucynta ER (Long Acting Opioid)
Is the patient naïve to long acting opioid therapy?
Yes
No
If Yes, has your patient experienced a treatment failure or adverse reaction to Nucynta IR (immediate release)?
Yes
No
Nucynta IR (Short Acting Opioid)
Has your patient experienced a treatment failure or adverse reaction to tramadol plus 1 other preferred short-acting opioid?
Yes
No
Tramadol ER
Has your patient experienced a treatment failure or adverse reaction to immediate release tramadol?
Yes
No
If no, is the prescriber willing to change?
Yes
No
ConZip/Ryzolt/Ultram ER
Has your patient experienced a treatment failure or adverse reaction to immediate release tramadol?
Yes
No
Has your patient experienced a treatment failure or adverse reaction to the generic, tramadol ER?
Yes
No
If no, is the prescriber willing to change?
Yes
No
Soma Products
Has your patient experienced a treatment failure or adverse reaction to 1 preferred analgesic plus 2 preferred skeletal muscle relaxants?
Yes
No
Revision Date: February 18, 2016
For billing questions, call 1-800-343-9000.
For clinical concerns or Preferred Drug Program questions, visit
www.nyhealth.gov and http://newyork.fhsc.com or call 1-877-309-9493.
Page 2
Magellan Medicaid Administration
Opioid Agents Prior Authorization Fax Form
Therapeutic Duplication
Two Long Acting Opioids (Applies to LAO, Tramadol ER products, and Methadone)
What is the clinical rationale for the patient requiring concurrent use of two or more long acting opioids?
Please list long acting opioid(s):
Opioid/Benzodiazepine (Applies to all SAO & LAO, Tramadol ER products, Methadone, Soma compound w/codeine, & Fentanyl
Mucosal Agents)
What is the clinical rationale for the patient requiring concurrent use of a benzodiazepine and an opioid?
Please list the Benzo product(s):
Opioid/Buprenorphine (Applies to all SAO & LAO, Tramadol ER products, Methadone, Soma compound w/codeine, & Fentanyl
Mucosal Agents)
Is the patient currently taking a Buprenorphine product?
Yes
No
Are you willing to prescribe a non-opiate analgesic (i.e. NSAID, etc.)?
Yes
No
Is the patient having surgery or had an acute event requiring narcotic pain medication?
Yes
No
What is the clinical rationale for the patient requiring concurrent use of an opioid and a buprenorphine product?
Frequency/Quantity/Duration (F/Q/D)
For all Short Acting and Long Acting Opioids
Does quantity prescribed exceed the recommended dosage? (Please refer to the PDL at newyork.fhsc.com)
Yes
No
If Yes, please provide clinical reason:
For Long Acting Opioids, Methadone and Tramadol ER products
For an initial fill for an opioid naïve patient, please provide a clinical rationale for requesting a long acting opioid in an opioid naïve
patient.
Does quantity prescribed exceed the per day limit? (Please refer to the PDL at newyork.fhsc.com)
Yes
No
If Yes, please provide clinical rationale for exceeding the FDA approved/Compendia supported quantity limit:
For Short Acting Opioids only
Medicaid limits the first fill of an opioid to a 15 day’s supply for opioid naïve patients. What is the clinical rationale for exceeding the 15
day’s supply initial fill duration limit?
For Short Acting Opioids only (for requests that exceed the 90 day duration limit)
Are you currently tapering the patient off their short-acting narcotic and attempting to utilize different treatment options?
Yes
No
Revision Date: February 18, 2016
For billing questions, call 1-800-343-9000.
For clinical concerns or Preferred Drug Program questions, visit
www.nyhealth.gov and http://newyork.fhsc.com or call 1-877-309-9493.
Page 3
Magellan Medicaid Administration
Opioid Agents Prior Authorization Fax Form
Frequency/Quantity/Duration (F/Q/D)
(Patients with a diagnosis of neuropathic pain) Has the patient attempted to utilize an anti-seizure or antidepressant medication FDA
approved for the management of neuropathic pain?
Yes
No
If no, is the prescriber willing to change to an anti-seizure or antidepressant medication FDA approved for the management of
neuropathic pain?
Yes
No
(Patients with a diagnosis of low back pain/musculoskeletal pain and request is for a single entity SAO) Has the patient attempted to
utilize acetaminophen or a non-steroidal anti-inflammatory drug?
Yes
No
If no, is the prescriber willing to change to acetominophen or a preferred NSAID?
Yes
No
Is the patient currently on a long acting opioid?
Yes
No
Is the long acting opioid being optimized?
Yes
No
Has your patient experienced a treatment failure or adverse reaction to a long acting opioid or is there a contraindication to using a long
acting opioid?
Yes
No
Has the patient’s risk for opioid misuse or abuse been assessed?
Yes
No
For Methadone (for requests that exceed max of #12 units per day , #360 units per 30 days)
Is Methadone being prescribed for the treatment of opioid addiction?
Yes
No
If yes, Methadone must be billed through a Methadone Maintenance Treatment Program.
Has the patient been assessed for clinical risks of opioid/substance abuse/addiction?
Yes
No
Does the patient have an underlying cardiovascular disorder or history of cardiac arrhythmias?
Yes
No
Will the patient periodically be clinically assessed for the need for gradual dosage adjustments?
Yes
No
What is the clinical rationale for the patient requiring a dose exceeding 120mg/day?
Yes
No
For Soma Compound with Codeine
Does quantity or duration prescribed exceed the recommended dosage? (Please refer to the PDL at newyork.fhsc.com)
Yes
No
If yes, does the patient belong to a patient population that is at increased risk for harm with carisoprodol containing products such as
patients over the age of 65 years, patients with history of drug/substance abuse or addiction, or patients that have concomitant use of
other CNS depressants?
Yes
No
What is the clinical rationale for exceeding the quantity/duration limit?
I attest that this is medically necessary for this patient and that all of the information on this form is accurate to the best of my knowledge. I attest that
documentation of the above diagnosis and medical necessity is available for review if requested by New York Medicaid.
PRESCRIBER’S SIGNATURE
Revision Date: February 18, 2016
DATE
For billing questions, call 1-800-343-9000.
For clinical concerns or Preferred Drug Program questions, visit
www.nyhealth.gov and http://newyork.fhsc.com or call 1-877-309-9493.
Page 4









