Silicone dielectric elastomers based on radical crosslinked high
advertisement

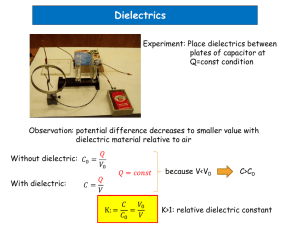
Supporting Information Silicone dielectric elastomers based on radical crosslinked high molecular weight polydimethylsiloxane co-filled with silica and barium titanate Adrian Bele, George Stiubianu, Cristian-Dragos Varganici, Mircea Ignat, Maria Cazacu Figure S1. Film formation procedure. Theoretical estimation of dielectric permittivity The following theoretical approaches were used for predicting the values of effective dielectric permittivity of polymer composite systems. 1. Volume-fraction average: εff = φpεp + φsεs + φbεb (1) where p, s and b are the siloxane polymer, the silica and the barium titanate phase, respectively, and φ is the volume fraction of the constituents, with φp = 1 – (φs + φb). This model (Equation 1) predicts a sharp increase of the effective dielectric constant starting at a low volume fraction of the ceramic filler. In fact, contradictory results were found in both theoretical [38] and experimental studies [39]. 2. The Maxwell-Garnett equation; it is an approximation of a single spherical inclusion surrounded by a continuous matrix of the polymer when the filler fraction goes to zero (infinite dilution) [40]. Since none of these conditions are fulfilled for the prepared elastomers – there is more than one filler and the fractions of the fillers are significant – therefore this equation can be used for calculating estimative values of εff only for samples containing just silica (S10 B0, S15 B0, S30 B0): εff = εp· [εs + 2εp – 2(1 – φp)(εp – εs)]/[εs + 2εp + (1 – φp)(εp – εs)] (2) 3. In the Bruggeman model, the binary mixture is made of repeated unit cells, each consisting of the polymer matrix which has spherical inclusions in the center [40]. The effective dielectric constant calculated with the Bruggeman equation increases sharply for filler volume fractions above 20%. The effective dielectric constant (εff) of the composite mixture [41] is given by: (εeff – εp)/(εeff + 2εp) = φs (εs – εp)/(εs + 2εp) + φb (εb – εp)/(εb + 2εp) (3) The Maxwell-Garnett equation (2) is just a particular case of the Bruggeman model. 4. The two-component Lichtenecker-Rother model [42] for complex permittivity is used for such calculations in different systems, such as air-particulate composites [43], ceramic-ceramic composites [44] and polymer-ceramic composites [45]. In this model the effective permittivity of two-component systems is determined by introducing the volume fraction of each component according to equation: εeffβ = φp εpβ + φs εsβ + φb εbβ (4) β is a dimensionless parameter and its value is determined by the shape and orientation of the filler particles within the bulk composite [46] and can take values between 1 and -1. Since the microparticles of silica and barium titanate can be described as spherical inclusions and randomly oriented ellipsoids, the value of β = 1/3, as determined by Landau, Lifshitz [47,48]. Figure S2 shows the values for the dielectric constant of the samples as resulted from the above formulas and can be easily compared with the experimental values. The values for εp=2.9 and εs=3.9 and εb=1700, density is 1 g/cm3 for the siloxane polymer, 2.2 g/cm3 for silica and 6.03 g/cm3 for barium titanate and the volume fractions are as follows: -φp=1, φs=0 and φb=0 for S0B0, -φp=0.9524, φs=0.0476 and φb=0 for S10 B0, -φp=0.949, φs=0.043 and φb=0.0078 for S10 B5, -φp=0.934, φs=0.042 and φb=0.0232 for S10 B15, -φp=0.936, φs=0.0637 and φb=0 for S15 B0, -φp=0.929, φs=0.0632 and φb=0.00771 for S15 B5, -φp=0.915, φs=0.0623 and φb=0.0227 for S15 B15, -φp=0.880, φs=0.120 and φb=0 for S30 B0, -φp=0.873, φs=0.119 and φb=0.00725 for S30 B5, -φp=0.861, φs=0.117 and φb=0.0214 for S30 B15. εp=2.9 ; εs=3.9 ; εb=1700 2 S0B0: φp=1, φs=0 and φb=0, S10 B0: φp=0.9524, φs=0.0476 and φb=0, S10 B5: φp=0.949, φs=0.043 and φb=0.0078, S10 B15: φp=0.934, φs=0.043 and φb=0.023, S15 B0: φp=0.936, φs=0.064 and φb=0, S15 B5: φp=0.929, φs=0.0632 and φb=0.00771, S15 B15: φp=0.915, φs=0.0623 and φb=0.0227, S30 B0: φp=0.880, φs=0.120 and φb=0, S30 B5: φp=0.873, φs=0.119 and φb=0.00725, S30 B15: φp=0.861, φs=0.117 and φb=0.0214. 0) Measured values 1) Volume fraction: εff = φpεp + φsεs + φbεb 2) Maxwell: εff = εp· [εs + 2εp – 2(1 – φp)(εp – εs)]/[εs + 2εp + (1 – φp)(εp – εs)] 3) Bruggeman: (εeff – εp)/(εeff + 2εp) = φs (εs – εp)/(εs + 2εp) + φb (εb – εp)/(εb + 2εp) 4) Lichtenecker-Rother: εeffβ = φp εpβ + φs εsβ + φb εbβ Table S1. Dielectric permittivity values predicted by using different models Sample Silica, Barium Measured Expected dielectric permittivity value wt% Titanate, dielectric Volume Maxwell permittivity fraction Garnett wt% Bruggeman LichteneckerRother value S0B0 0 0 3.02 2.90 2.90 2.90 2.90 S10 B0 10 0 3.41 2.95 2.94 2.94 2.95 S10 B5 10 5 3.67 16.18 - 3.01 3.46 S10 B15 10 15 3.95 41.89 - 3.14 3.69 S15 B0 15 0 3.48 2.96 2.96 3.15 2.98 S15 B5 15 5 3.66 16.04 - 3.01 3.48 S15 B15 15 15 4.09 41.48 - 3.16 4.68 S30 B0 30 0 3.45 3.02 3.01 3.01 3.01 S30 B5 30 5 3.89 15.32 - 3.07 3.50 S30 B15 30 15 4.26 39.33 - 3.2 4.63 3 Figure S2. Plotting experimental dielectric permittivity values as compared with those theoretical estimated by using different models. 4 a b 5 c d Figure S3. DSC curves for: a- PDMS and series S0; b – series S10; c – series S15; d – series S30 (H1 – first heating; H2 – second heating; C – cooling). 6 Figure S4. Water vapor sorption isotherms recorded at room temperature. 7 S10B0 S10B5 S10B15 8 S15B0 S15B5 S15B15 9 S30B0 S30B5 S30B15 Figure S5. The electric responses at an applied mechanical impulse. 10 References 38. Ying KL, Hsieh TE (2007) Sintering behaviors and dielectric properties of nanocrystalline barium titanate. Mater Sci Eng B Solid-State Mater Adv Technol 138:241–245. doi: 10.1016/j.mseb.2007.01.002 39. Brosseau C (2006) Modelling and simulation of dielectric heterostructures: a physical survey from an historical perspective. J Phys D Appl Phys 39:1277–1294. doi: 10.1088/00223727/39/7/S02 40. Yoon DH, Zhang J, Lee BI (2003) Dielectric constant and mixing model of BaTiO3 composite thick films. Mater Res Bull 38:765–772. doi: 10.1016/S0025-5408(03)00075-8 41. Bosch S, Ferré-Borrull J, Leinfellner N, Canillas A (2000) Effective dielectric function of mixtures of three or more materials: a numerical procedure for computations. Surf Sci 453:9– 17. doi: 10.1016/S0039-6028(00)00354-X 42. Lichtenecker K, Rother K (1931) Die Herleitung des logarithmischen Mischungsgesetz es aus allgemeinen Prinzipien der stationären Strömung. Physikalische Zeitschrift 32:255-260 43. Nelson SO (1983) Observations on the Density Dependence of Dielectric Properties of Particulate Materials. J. Microw. Power 18:143-152 44. Landau L, Lifshitz E (1984.) Electrodynamics of Continuous Media 2nd edn. Pergamon Press, New York 45. Looyenga H (1965) Dielectric constants of heterogeneous mixtures. Physica 31:401–406. doi: 10.1016/0031-8914(65)90045-5 46. Karkkainen KK (2000) Effective permittivity of mixtures: numerical validation by the FDTD method. IEEE Trans Geosci Remote Sens 38:1303–1308. doi: 10.1109/36.843023 47. Brosseau C, Quéffélec P, Talbot P (2001) Microwave characterization of filled polymers. J Appl Phys 89:4532–4540. doi: 10.1063/1.1343521 48. Gershon D, Calame JP, Birnboim a. (2001) Complex permittivity measurements and mixings laws of alumina composites. J Appl Phys 89:8110–8116. doi: 10.1063/1.1369400 11