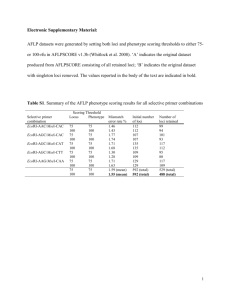
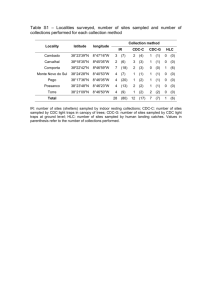
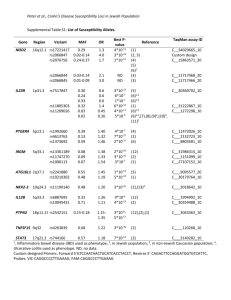
N=2900 spaces are counted

Immunochip-based analysis of 72.000 individuals identifies 50 novel IBD loci, refining definitions of disease pathways.
R.K.Weersma on behalf of the International IBD Genetics Consortium (IIBDGC)
INTRODUCTION: A seminal finding of the genome-wide association study (GWAS) era is the identification of a marked overlap of loci between immune-mediated diseases. The Immunochip Consortium designed a customized chip with ≈200.000 single nucleotide polymorphisms focused on: 1) fine-mapping 190 known loci for 9 different immune-mediated diseases and; 2) follow up of top 2000 UC and CD GWAS signals not previously studied
METHODS: A meta-analysis was performed combining HapMap3 imputed CD and UC GWAS data with a larger case-control cohort genotyped on the Immunochip. Altogether, 19,416 CD, 17,016 UC and 36,602 controls, all of European ancestry were analyzed
RESULTS: We identified 50 novel genomic regions with genome-wide significant association (P-value < 5 x
10-8), bringing the total number of IBD loci to 149. These new associations further implicate Th17 cells
(RORC, the Th17 master transcription factor), the Th1 pathway (STAT4, involved in IL-12 signaling; IFNGR2, interferon-gamma receptor 2), co-stimulation (ITGAL, integrin alpha-L/LFAy (LITAF, lipopolysaccharide induced TNF factor), NF-kB signaling (NFKB1/p50; TRAF3IP2), the KIR-family (KIR3DL3, killer cell immunoglobulin-like receptor), and the NOD2 pathway (CD-specific association to RIPK2, the
NOD2 signaling partner). Previously identified UC loci are now also confirmed CD loci (IL2/IL21,
IFNg/IL22/IL26, FCGR2A); conversely, known CD loci now demonstrate association with UC (PTPN2,
LRRK2, IKZF1). The large sample size provides power to fine-map loci and define differential effect sizes. A range of CD-specific, UC-specific and IBD-general effects is observed. Among autophagy genes, while the
ATG16L1 association is CD-specific, significant associations are observed for IRGM in both CD and UC, albeit with more modest effect sizes in UC compared to CD. The IFNGR2 association is CD-specific; distinct, loss-of-function mutations in IFNGR2 have been associated with increased susceptibility to mycobacterial infections. Multiple loci previously been associated with other immune-mediated diseases including rheumatoid arthritis, celiac disease, multiple sclerosis, type 1 diabetes, psoriasis and SLE are now also associated with CD (CLEC16A, SLC12A5, ANKRD55, CD40, STAT4, JAZF1) and UC (C5orf62,
PSORS1C1).
CONCLUSIONS: Immune-mediated diseases are associated with numerous loci that are often shared between disease subtypes. Distinct phenotypes are distinguished by different combinations of loci, variable effect sizes and distinct allelic architectures within loci. Future genetic and genomic studies focused on these loci will facilitate therapeutic interventions across these diseases.