Changes in the electronic Immunisation Handbook – 6 June 2014
advertisement

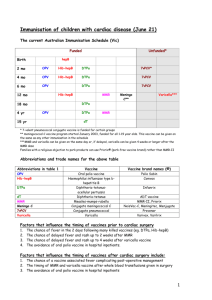
Changes in the electronic Immunisation Handbook – 6 June 2014 Since the print edition of the Immunisation Handbook was published in May 2014, changes have been made to the electronic edition of the Handbook to reflect confirmed funding decisions. Immunisation providers are encouraged to ensure that they are using the current recommendations as available in the electronic edition of the Handbook, and to refer to the Pharmaceutical Schedule (www.pharmac.health.nz) for the number of funded doses and any subsequent changes to the funding decisions. Summary of the changes Schedule vaccines In addition to DTaP-IPV vaccine, DTaP-IPV-HepB/Hib vaccine may be administered to children aged under 10 years for catch-up immunisation. All vaccines on the National Immunisation Schedule are funded for (re-)vaccination of individuals following significant immunosuppression. IPV vaccine may be used for primary immunisation of unvaccinated or partially-vaccinated individuals. Td vaccine may be used for primary vaccination of unvaccinated or partially-vaccinated individuals, and may be used for boosting of tetanus-prone wounds. Vaccines for special groups Meningococcal, pneumococcal or varicella vaccines will be funded for the following individuals. Meningococcal conjugate vaccines (MenCCV and MCV4-D) individuals pre- or post-splenectomy or with functional asplenia individuals with HIV, complement deficiency (acquired, including monoclonal therapy against C5, or inherited) or pre- or post-solid organ transplant close contacts of meningococcal cases bone marrow transplant patients individuals following immunosuppression. Pneumococcal conjugate vaccine (PCV13) high-risk children who have previously received four doses of PCV10 (re-)vaccination for children aged under 18 years: with HIV; who are post-haematopoietic stem cell transplant (HSCT) or chemotherapy; who are pre- or post-splenectomy or with functional asplenia; who are pre- or post-solid organ transplant, renal dialysis and other severely immunosuppressive regimens. Pneumococcal polysaccharide vaccine (23PPV) individuals who are pre- or post-splenectomy or with functional asplenia high risk children aged under 18 years. Varicella vaccine non-immune patients: o with chronic liver disease who may in future be candidates for transplantation o with deteriorating renal function prior to transplantation o prior to solid organ transplant o prior to any elective immunosuppression patients at least two years after bone marrow transplantation, on advice of their specialist patients at least six months after completion of chemotherapy, on advice of their specialist HIV-positive individuals with mild or moderate immunosuppression who are non-immune to varicella, on advice of their HIV specialist individuals with inborn errors of metabolism at risk of major metabolic decompensation, with no clinical history of varicella household contacts of paediatric patients who are immune compromised, or undergoing a procedure leading to immune compromise, where the household contact has no clinical history of varicella household contacts of adult patients who have no clinical history of varicella and who are severely immune compromised or undergoing a procedure leading to immune compromise, where the household contact has no clinical history of varicella. Detailed description of the changes The changes made in each section of the Handbook have been bolded in the text below for emphasis. Introduction National Immunisation Schedule 2014 Point 6 has been replaced with: 6. DTaP-IPV-HepB/Hib (paediatric diphtheria, tetanus, acellular pertussis, polio, hepatitis B and Hib vaccine, Infanrix-hexa) and DTaP-IPV may be administered to children aged under 10 years for catch-up immunisation. This is a change from the previous recommendation of age 7 years (see Appendix 2: ‘Planning immunisation catch-ups’ and the relevant disease chapters). Point 8 has been added: 8. All vaccines on the National Immunisation Schedule are funded for (re-) vaccination of individuals following significant immunosuppression. The timing and number of doses should be discussed with the individual’s specialist. 2014 targeted programmes for special groups Point 5 has been replaced with: 5. Meningococcal conjugate vaccines, MenCCV and MCV4-D (see chapter 12), will be funded for: individuals pre- or post-splenectomy or with functional asplenia individuals with HIV, complement deficiency (acquired, including monoclonal therapy against C5, or inherited) or pre- or post-solid organ transplant close contacts of meningococcal cases bone marrow transplant patients individuals following immunosuppression. Point 6 has been added: 6. Pneumococcal conjugate vaccine, PCV13 (see chapter 15) will be funded for: high-risk children who have previously received four doses of PCV10 (re-)vaccination for children aged under 18 years: with HIV; who are posthaematopoietic stem cell transplant (HSCT) or chemotherapy; who are pre- or post-splenectomy or with functional asplenia; who are pre- or post-solid organ transplant, renal dialysis and other severely immunosuppressive regimens. Point 7 has been added: 7. Pneumococcal polysaccharide vaccine, 23PPV (see chapter 15), will be funded for: individuals who are pre- or post-splenectomy or with functional asplenia high risk children aged under 18 years. Point 8 has been replaced with: 8. Varicella vaccine (see chapter 21) will be funded for the following groups: non-immune patients: – with chronic liver disease who may in future be candidates for transplantation – with deteriorating renal function prior to transplantation – prior to solid organ transplant – prior to any elective immunosuppression patients at least two years after bone marrow transplantation, on advice of their specialist patients at least six months after completion of chemotherapy, on advice of their specialist HIV-positive individuals with mild or moderate immunosuppression who are non-immune to varicella, on advice of their HIV specialist individuals with inborn errors of metabolism at risk of major metabolic decompensation, with no clinical history of varicella household contacts of paediatric patients who are immune compromised, or undergoing a procedure leading to immune compromise, where the household contact has no clinical history of varicella household contacts of adult patients who have no clinical history of varicella and who are severely immune compromised or undergoing a procedure leading to immune compromise, where the household contact has no clinical history of varicella. Note that ‘post-exposure prophylaxis for immune competent inpatients’ has been removed from the ‘non-immune patients’ section. Table 2: Funded vaccines for special groups The meningococcal, pneumococcal and varicella rows have been replaced with: Meningococcal conjugate vaccines (see chapter 12) Meningococcal conjugate vaccines, MenCCV and MCV4-D, should be offered to: individuals pre- or post-splenectomy or with functional asplenia individuals with HIV, complement deficiency (acquired, including monoclonal therapy against C5, or inherited) or pre- or post-solid organ transplant close contacts of meningococcal cases bone marrow transplant patients individuals following immunosuppression. PCV13 for: Pneumococcal conjugate (PCV13) and pneumococcal high risk children who have previously received 4 doses of PCV10 polysaccharide (23PPV) (re-)vaccination for children aged under 18 years: with HIV; who are vaccines (see chapter 15) post-haematopoietic stem cell transplant (HSCT) or chemotherapy; who are pre- or post-splenectomy or with functional asplenia; who are preor post-solid organ transplant, renal dialysis and other severely immunosuppressive regimens. 23PPV for: individuals who are pre- or post-splenectomy or with functional asplenia high-risk children aged under 18 years. Varicella vaccine (see chapter 21) Recommended for: non-immune patients: – with chronic liver disease who may in future be candidates for transplantation – with deteriorating renal function before transplantation – prior to solid organ transplant – prior to any elective immunosuppression* patients at least two years after bone marrow transplantation, on advice of their specialist patients at least six months after completion of chemotherapy, on advice of their specialist HIV-positive individuals with mild or moderate immunosuppression who are non-immune to varicella, on advice of their HIV specialist individuals with inborn errors of metabolism at risk of major metabolic decompensation, with no clinical history of varicella household contacts of paediatric patients who are immune compromised, or undergoing a procedure leading to immune compromise, where the household contact has no clinical history of varicella household contacts of adult patients who have no clinical history of varicella and who are severely immunocompromised or undergoing a procedure leading to immune compromise, where the household contact has no clinical history of varicella. Chapter 2 –Processes for safe immunization 2.2.4 Immunisation consent in primary care The National Immunisation Register leaflet (HE1501) has been removed. 2.7 Adult vaccination Table 2.11 Checklist for adult vaccination, excluding travel The meningococcal, pneumococcal, IPV and varicella rows have been replaced with: Meningococcal conjugate (chapters 4 and 12) For individuals: pre- or post-splenectomy or with functional asplenia with HIV, complement deficiency (acquired, including monoclonal therapy against C5, or inherited) or pre- or post-solid organ transplant who are close contacts of meningococcal cases bone marrow transplant patients following immunosuppressiona Pneumococcal conjugate and 23PPV for individuals pre- or post-splenectomy or with functional asplenia polysaccharide (chapters 4 and 15) IPV (chapter 16) Any unvaccinated or partially-vaccinated individual Varicella (chapter 21) Non-immune individuals: with chronic liver disease with deteriorating renal function before transplantation prior to solid organ transplant prior to any elective immunosuppressiona Patients at least 2 years after bone marrow transplant Patients at least 6 months after completion of chemotherapy HIV-positive patients who are non-immune to varicella, with mild or moderate immunosuppression Individuals with inborn errors of metabolism at risk of major metabolic decompensation, with no clinical history of varicella Household contacts of paediatric patients who are immune compromised or undergoing a procedure leading to immune compromise, where the household contact has no clinical history of varicella Household contacts of adult patients who have no clinical history of varicella and who are severely immune compromised, or undergoing a procedure leading to immune compromise, where the household contact has no clinical history of varicella Chapter 3 – Vaccination questions and concerns 3.13 Allergies and illnesses What if the child is due to have an operation (elective surgery)? Varicella vaccine has been added to the third paragraph: Pneumococcal, meningococcal, Hib, influenza and varicella vaccines are recommended for these individuals pre- or post-splenectomy (see section 4.3.4 and the relevant disease chapters). Chapter 4 – Immunisation of special groups 4.2.7 Infants with HIV Meningococcal vaccine has been added: Infants with HIV infection who do not have severe immunosuppression should follow the routine Schedule and are also eligible to receive funded meningococcal, varicella and influenza vaccines. 4.2.8 Other conditions Funded varicella vaccine has been added to the second bullet point. Varicella vaccine is funded for infants with inborn errors of metabolism at risk of major metabolic decompensation (see section 21.5); and is recommended for a variety of endocrine disorders – discuss with the specialist. 4.3.1 Introduction Household contacts ‘Paediatric’ has been removed from the last sentence of the second paragraph about household contacts: Varicella vaccine is funded for household contacts of patients who are immunocompromised or undergoing a procedure leading to immunocompromise. 4.3.2 Primary immune deficiencies A new second paragraph has been added: Hib, PCV13 and Td vaccines may be used in testing for primary immune deficiencies, on the recommendation of an internal medicine physician or paediatrician. 4.3.3 Secondary (acquired) immune deficiencies Solid organ transplants Funded pneumococcal vaccine has been added to the first sentence of the final paragraph: In patients undergoing organ transplantation, pneumococcal vaccine (funded for children aged under 18 years) should be given at least two weeks before the transplant. HIV infection – Table 4.4 Additional vaccine recommendations (funded and unfunded) for HIV-positive individuals The meningococcal conjugate vaccine rows have been shaded, to reflect funding of these vaccines for HIV-positive individuals (vaccinators are reminded to refer to the Pharmaceutical Schedule for the number of funded doses). MCV4-D has been added to the list of funded vaccines for adults aged 18 years and older. PCV13 is now funded for HIV-positive children aged 5 to under 18 years, so this row has been shaded in the table. 4.3.4 Asplenia Table 4.5 Additional vaccine recommendations (funded and unfunded) and schedules for individuals with functional or anatomical asplenia Functional asplenia has been added to the ‘Adults ≥18 years’ row. A new footnote ‘h’ has been added to the Hib row, as Hib vaccine is not funded for adults with functional asplenia. 4.3.6 (Re-)vaccination following immunosuppression A reminder about the period of immunosuppression has been added: (Note that the period of immunosuppression due to steroid or other immunosuppressive therapy must be longer than 28 days.) Chapter 9 – Human papillomavirus (HPV) References The URL to reference 7 has been replaced with: https://cdn.auckland.ac.nz/assets/fmhs/faculty/ahrg/docs/2012-overview.pdf Chapter 12 – Meningococcal disease Key information The funded vaccine indications have been replaced with: Funded vaccine indications MCV4-D or MenCCV for individuals: pre- or post-splenectomy or with functional asplenia with HIV, complement deficiency (acquired, including monoclonal therapy against C5, or inherited) or pre- or post-solid organ transplant who are close contacts of meningococcal cases who are bone marrow transplant patients following immunosuppression. 12.5.1 At-risk individuals Table 12.5 Meningococcal group C conjugate (MenCCV) and quadrivalent meningococcal vaccine (MCV4-D) recommendations The table has been updated to reflect the new funding. Recommended and funded MenCCV and MCV4-D are recommended and funded for individuals: who are pre- or post-splenectomy or with functional aspleniaa,b with HIV, complement deficiency (acquired, including monoclonal therapy against C5, or inherited) or who are pre- or post-solid organ transplantb who are close contacts of meningococcal cases who are bone marrow transplant patientsb following immunosuppression.b,c Recommended but not funded MenCCV and MCV4-D are recommended,d but not funded, for individuals: who are laboratory workers regularly handling meningococcal cultures who are travelling to high-risk countries (see the WHO website), or before the Hajj who are adolescents and young adults living in communal accommodation (eg, in a hostel or at boarding school, in military accommodation, in correctional facilities or in other long-term institutions). a Pneumococcal, Hib, influenza and varicella vaccines are also recommended for individuals pre- or post-splenectomy or with functional asplenia. See section 4.3.4. b See sections 4.2 and 4.3 for more information. c The period of immunosuppression due to steroid or other immunosuppressive therapy must be longer than 28 days. d Quadrivalent meningococcal polysaccharide vaccines are another option for individuals aged 2 years and older. Chapter 14 – Pertussis 14.5.1 Children Catch-up immunisation The bullets have been updated to reflect the DTaP-IPV-HepB/Hib funding for children aged under 10 years: DTaP-IPV-HepB/Hib or DTaP-IPV may be used for primary immunisation of children aged under 10 years. Tdap may be used for primary immunisation of children aged 7 to under 18 years. Chapter 15 – Pneumococcal disease Key information The ‘Funded vaccines’ and ‘Funded immunisation’ schedule rows have been replaced with: Funded vaccines 13-valent protein conjugate vaccine, PCV13 (Prevenar 13): for all children aged under 5 years. PCV13: for high-risk children who have previously received 4 doses of PCV10; for (re-)vaccination of children aged under 18 years with HIV, who are post-haematopoietic stem cell transplant (HSCT) or chemotherapy, who are pre- or post-splenectomy or with functional asplenia, who are pre- or post-solid organ transplant, renal dialysis and other severely immunosuppressive regimens. 23-valent polysaccharide vaccine, 23PPV (Pneumovax 23): for individuals who are pre-or post-splenectomy or with functional asplenia; for high-risk children aged under 18 years. Funded immunisation schedule Children who have started with PCV10 can continue with PCV13. Healthy children aged under 5 years: PCV13 at ages 6 weeks, 3, 5 and 15 months. High-risk children aged under 5 years: standard PCV13 schedule, plus 1 dose of 23PPV at age 2 years or older (with at least 8 weeks between the last PCV13 and the 23PPV). If risk persists, revaccinate once with 23PPV, 5 years after the first 23PPV. Eligible children aged 5 to under 18 years: 1 dose of PCV13 followed 8 weeks later with 1 dose of 23PPV. Revaccinate once with 23PPV, 5 years after the first 23PPV. Eligible adults: a maximum of 3 doses of 23PPV in their lifetime, a minimum of 5 years apart. 15.4.1 Available vaccines Funded vaccines The following has been added to the end of the first bullet point: or who are eligible for (re-)vaccination. 15.5.2 High-risk children aged under 5 years PCV13 The following has been added to the end of the paragraph: High-risk children who have previously received four doses of PCV10 may receive one dose of PCV13. 15.5.3 Older children and adults at higher risk of pneumococcal disease The note has been deleted: Note: Only children aged under 18 years with functional asplenia or who are pre- or post-splenectomy are eligible for funded PCV13 and 23PPV vaccines. Adults aged 18 years and older who are pre- or post-splenectomy are eligible for funded 23PPV vaccine. However, PCV13 is recommended (but not funded) for adults. 15.5.4 (Re-)vaccination HIV has been added to the list of conditions. 15.5.5 Summary of pneumococcal vaccine schedules Table 15.5 Summary of pneumococcal vaccine recommendations (funded and unfunded) and schedules The table has been updated as shown below. Note that ‘Children aged 5 to <18 years with other highrisk conditions’ has been moved from the ‘Recommended but not funded’ section to the ‘Funded and unfunded’ section, to reflect the funded 23PPV vaccine. High-risk children aged 5 to under 18 years (funded and unfunded) Children aged 5 to <18 1 dose of PCV13.b,e years with functional Followed by 1 dose of 23PPV at least 8 weeks after the PCV13 dose. asplenia or who are pre- or Revaccinate once with 23PPV, 5 years after the 1st 23PPV. post-splenectomy,a,d or who meet the PCV (re-) vaccination criteria Children aged 5 to <18 years with other highrisk conditions 1 dose of PCV13.b,e 1 dose of 23PPV at least 8 weeks after the PCV13 dose. Revaccinate once with 23PPV, 5 years after the 1st 23PPV. High-risk adults aged 18 years (funded and unfunded) Adults (≥18 years) who are 1 dose of PCV13.b,e pre- or post-splenectomya,d Give a maximum of 3 doses of 23PPV in a lifetime, a minimum of 5 years apart. or with functional The 1st 23PPV dose is given at least 8 weeks after PCV13; the 2nd a minimum of asplenia 5 years later; the 3rd dose at age ≥65 years. Chapter 16 – Poliomyelitis 16.5.3 Unimmunised adults and children The first paragraph has been reworded to reflect funding for partially immunised individuals. For partially immunised or previously unimmunised individuals, a primary immunisation course consists of three doses of IPV-containing vaccine (funded). Chapter 19 – Tetanus 19.5.2 Adults and children from age 10 years The second paragraph has been reworded to reflect funding for partially immunised individuals. For partially immunised or previously unimmunised individuals aged 10 years and older, a primary immunisation course consists of three doses of a tetanus toxoid-containing vaccine at intervals of not less than four weeks (see Appendix 2). Table 19.2 Guide to tetanus prophylaxis in wound management Footnote a has been updated to reflect funding up to age 10 years for DTaP-IPV and DTaP-IPVHepB/Hib vaccines: a See Appendix 2 for catch-up schedules for previously unimmunised children. DTaPcontaining vaccine may be used in children aged under 10 years. 19.5.4 (Re-)vaccination Has been replaced with: Tetanus toxoid-containing vaccine is funded for (re-)vaccination following immunosuppression. (See also sections 4.2 and 4.3.) Chapter 21 – Varicella 21.5.1 Funded vaccine for high-risk groups Table 21.1 High-risk groups eligible for funded varicella immunisation The table has been updated to reflect the new funding. Note that ‘post-exposure prophylaxis for immune competent inpatients’ has been removed from the ‘non-immune patients’ section. High-risk groups funded for immunisation are: non-immune patients: – with chronic liver disease who may in future be candidates for transplantation – with deteriorating renal function before transplantation – prior to solid organ transplant – prior to any elective immunosuppression* patients at least 2 years after bone marrow transplantation, on the advice of their specialist patients at least 6 months after completion of chemotherapy, on the advice of their specialist HIV-positive patients who are non-immune to varicella, with mild or moderate immunosuppression, on the advice of an HIV specialist individuals with inborn errors of metabolism at risk of major metabolic decompensation, with no clinical history of varicella household contacts of paediatric patients who are immune compromised or undergoing a procedure leading to immune compromise, where the household contact has no clinical history of varicella household contacts of adult patients who have no clinical history of varicella and who are severely immune compromised or undergoing a procedure leading to immune compromise, where the household contact has no clinical history of varicella. * Note that the period of immunosuppression due to steroid or other immunosuppressive therapy must be longer than 28 days. 21.5.4 Immunosuppressed individuals The first sentence of the second paragraph has been updated to reflect funding for household contacts of immunosuppressed individuals. Where immunosuppressed individuals cannot be vaccinated, it is important to vaccinate the household members and other close contacts (funded for household contacts) to provide ‘ring fence’ protection (see sections 4.2, 4.3 and 21.7). Appendix 2 A2.1.1 Principles of catch-up for children aged under 10 years Point 6 has been replaced with: 6. For infants and children aged under 10 years, use DTaP-IPV-HepB/Hib or DTaP-IPV for primary immunisation. Tdap may be used for primary immunisation of children aged 7 to under 18 years (note that Tdap is not registered for children aged under 10 years or for primary immunisation, but there are not expected to be any safety concerns). A2.1.3 National Immunisation Schedule catch-up guides for infants and children aged up to 18 years Table A2:7 Age at presentation: 5 years to under 10 years The table has been updated to reflect funding of both DTaP-IPV and DTaP-IPV-HepB/Hib for catchup immunisation of children aged under 10 years. Dose Vaccines First dose DTaP-IPV-HepB/Hiba or DTaP-IPVb Hep Bc MMR 4 weeks later DTaP-IPV-HepB/Hiba,d or DTaP-IPVb,d Hep Bc MMR 4 weeks later DTaP-IPV-HepB/Hiba,d 6 months later DTaP-IPVd or DTaP-IPVb,d Hep Bc Once the child has received the appropriate vaccines for their age, continue on the Schedule as usual. a Parents/guardians should be informed that their child will receive extra doses of Hib but there are no safety concerns with these extra doses. b If the parents/guardians prefer, vaccinators may administer the DTaP-IPV and Hep B vaccines as 2 separate injections instead of the combination DTaP-IPV-HepB/Hib vaccine. c Hep B is not required if DTaP-IPV-HepB/Hib is given. d If a child turns 10 years before completing their catch-up programme, they should continue on the 10 to under 18 years catch-up schedule (refer to Table A2.8). A2.2 Immunisation catch-up for adults aged 18 years and older Table A2.9: Primary immunisation requirements for adults aged 18 years and older Footnotes a and b have been updated to reflect funding of Td and IPV vaccines for partially immunised adults. a A primary course of 3 doses of adult Td vaccine is recommended and funded for unimmunised or partially-immunised adults. Unfunded Tdap may be offered as an alternative to Td for pertussis protection. At ages 45 and 65 years, the Td booster immunisation administration (the immunisation benefit) is not funded, although the vaccine is free. b A primary course of 3 doses of IPV is recommended and funded for unimmunised or partially-immunised adults. The minimum recommended interval between IPV doses 1 and 2 is 4 weeks; the 3rd IPV dose should be given at least 6 months after dose 2. If necessary, the interval may be shortened to 4 weeks between doses, but this is not the preferred schedule. Appendix 9 – Websites International websites American Academy of Pediatrics The name of the first article has been corrected: ‘Why immunize your child?’ Funded vaccines for special groups Note: this summary page is only in the print and PDF versions of the Handbook (at the back of the book, page 680). It is not in the e-book or html versions. A separate PDF of this table is available for download, to replace page 680 (http://www.health.govt.nz/system/files/documents/publications/funded-vaccines-for-specialgroups-june14.pdf). The meningococcal, pneumococcal and varicella rows have been updated to: MenCCV and For individuals: pre- or post-splenectomy or with functional asplenia; with HIV, complement MCV4-D deficiency (acquired or inherited) or pre- or post-solid organ transplant; close contacts of meningococcal cases; bone marrow transplant patients; following immunosuppression. PCV13 and 23PPV PCV13 for high-risk children who have previously received 4 doses of PCV10. PCV13 for children aged 5 to <18 years who are eligible for (re-) vaccination. 23PPV for individuals pre- or post-splenectomy or with functional asplenia; for high-risk children aged under 18 years. Varicella Non-immune patients: with chronic liver disease who may need a transplant in the future; with deteriorating renal function before transplant; prior to solid organ transplant; prior to elective immunosuppression. Patients at least 2 years after bone marrow transplant or at least 6 months after completion of chemotherapy, on advice of their specialist. HIV-positive individuals with mild or moderate immunosuppression who are non-immune to varicella, on advice of their specialist. Individuals with inborn errors of metabolism at risk of major metabolic decompensation, with no clinical history of varicella. Household contacts of paediatric patients who are immune compromised, or undergoing a procedure leading to immune compromise, where the household contact has no clinical history of varicella. Household contacts of adult patients who have no clinical history of varicella and who are severely immune compromised or undergoing a procedure leading to immune compromise, where the household contact has no clinical history of varicella.