Kinetic Molecular Theory in Action
advertisement

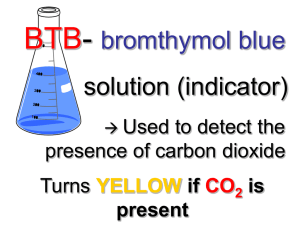
Kinetic Molecular Theory in Action Background: Kinetic Theory: The tiny particles in all forms of matter are in constant motion. Basic Assumptions: a. b. c. A gas is composed of particles, usually molecules or atoms. These particles are considered to be small, hard spheres that have insignificant volume and are relatively far apart from one another. The particles in a gas move rapidly in constant motion. They travel in straight paths and move independently of each other. As a result, gases fill their containers, regardless of the shape and volume of the containers; certain gases diffuse into space without limit. All collisions are considered perfectly elastic. This means that during collisions, kinetic energy is transferred without loss from one particle to another and the total kinetic energy remains constant. Safety: Wear safety goggles and apron while in the lab area. Wash all leftover chemical down the sink with plenty of water. Wash your hands after completing the lab. Clean up your entire lab work area. BTB will stain your skin and clothing. Purpose: To observe color changes in the chemical reactions of gases and to interpret these changes in terms of kinetic theory. Materials: Well plate with lid Cotton swabs Chemicals: Bromothymol blue BTB Sodium hydrogen sulfite NaHSO3 Ammonium chloride NH4Cl Potassium iodide KI Colored Pencils Hydrochloric acid HCl Sodium nitrite NaNO2 Sodium hydroxide NaOH Prelab: 1. Read pages 205-206 in your textbook and take notes in your lab notebook 2. Rearrange PV=nRT for P; V; n; R; T Procedure: 3. Add two drop of BTB to all of the wells EXCEPT the two center-most wells in your well plate. 4. Into the two center-most wells, add 2 drops HCl. 5. Into the two center-most wells, also add 2 drops of NaHSO3. QUICKLY place the lid onto your well plate. 6. Wait and observe. 7. Answer questions #1-4 (Use colored pencils where necessary). 8. When the reaction is complete, open the lid of the well plate. 9. Absorb the contents of the two center-most wells by using a Q-tip. 10. Into the two center-most wells, add 2 drops NaOH. 11. Into the two center-most wells, also add 2 drops of NH4Cl. QUICKLY place the lid onto your well plate. 12. Wait and observe. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. Answer questions #5 - 7 (Use colored pencils where necessary). When the reaction is complete, empty your well plate into the disposal bucket. Rinse the well plate thoroughly with distilled water. Add two drop of KI to all of the wells EXCEPT the two center-most wells in your well plate. Into the two center-most wells, add 2 drops NaNO2. Into the two center-most wells, also add 2 drops of HCl. QUICKLY place the lid onto your well plate. Wait and observe. Answer questions#8 – 10 (Use colored pencils where necessary). When the reaction is complete, empty your well plate into the disposal bucket. Rinse the well plate thoroughly with distilled water. Leave the well plate and lid on your lab table. Do NOT put it into your lab drawer. Analysis: 1. Describe in detail the changes that you observed in the drops of BTB over time. 2. Draw a picture illustrating the changes in BTB as observed from the top of your well plate. Draw a picture illustrating the changes in BTB as observed from the side of your well plate. (Yes, you will have two drawings here). Use colored pencils. 3. The color of the BTB drops changed even though you didn’t add a chemical directly to them. If the mixture in the two center-most wells produced a gas, would this explain the changes in the drops of BTB? Use the kinetic molecular theory to explain your answer in detail. 4. Translate the following word equation in a balanced chemical equation: Sodium hydrogen sulfite reacts with hydrochloric acid to produce sulfur dioxide gas , water and sodium chloride. (Include state of matter) 5. Describe in detail the changes that you observed in the drops of BTB over time. 6. Draw a picture illustrating the changes in BTB as observed from the top of your well plate. Draw a picture illustrating the changes in BTB as observed from the side of your well plate. (Yes, you will have two drawings here). Use colored pencils. 7. Translate the following word equation into a balanced chemical equation. Ammonium chloride reacts with sodium hydroxide to produce ammonia gas, NH3, water and sodium chloride. 8. Describe in detail the changes that you observed in the drops of BTB over time. 9. Draw a picture illustrating the changes in KI as observed from the top of your well plate. Draw a picture illustrating the changes in KI as observed from the side of your well plate. (Yes, you will have two drawings here). Use colored pencils. 10. Translate the following word equation into a balanced chemical equation. Sodium nitrite reacts with hydrochloric acid to produce nitrogen monoxide gas, water, sodium nitrate and sodium chloride.