ws15.4
advertisement

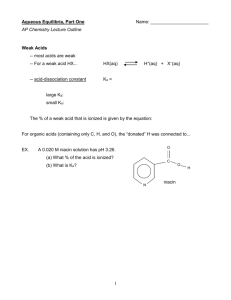
Chapter 15 Worksheet 4 (ws15.4) Acid-Base Properties of Salt Solution, Polyprotic Acids All acid-base reactions produce another acid and another base. The reaction between the stronger acid and the stronger base is called a “neutralization reaction”. Neutralization reactions produce a salt (an ionic compound) and (sometimes) water. (The definition of “salt” is an ionic compound produced by a neutralization reaction!) Most neutralization reactions go to completion. (When do they not go to completion?) Salt solutions can be neutral, acidic, or basic depending on the acid-base properties of the resulting ions: Cations (+): Most cations (+) such as ammonium ion and metal ions are weak acids. Metal ions are Lewis acids. However, the cations formed by group 1A (alkali metals) and the larger group 2A elements (Ca2+, Sr2+, Ba2+, Ra2+) have negligible acid strength. The presence of group I or II ions in a solution do not influence the pH. Anions (−): Anions derived from weak acids are weak bases (Kb=Kw/Ka). Anions derived from polyprotic acids are amphoteric (both weak acid and weak base). Anions derived from strong acids (eg Cl-) have negligible base strength so they do not influence the pH. 1. Predict whether each of the following salt solutions will be neutral, acidic, or basic. (Consider the acid-base properties of each ion.) Remember that ionic compounds dissociate completely in water. a. NaCl (aq) b. NH4NO3 (aq) c. NaCH3CO2 (aq) d. NH4NO2 (aq) e. AlCl3 (aq) 1 2. For each of the following salts, write the balanced molecular (overall) and net ionic equations for a neutralization reaction that would produces it. a. NaCl (aq) b. NH4NO3 (aq) c. NaCH3CO2 (aq) d. NH4NO2 (aq) 3. Fill in the blanks with either “neutral”, “acidic”, or “basic”. The reaction of a strong acid with a strong base produces a ______________ salt. The reaction of a strong acid with a weak base produces a ______________ salt. The reaction of a weak acid with a strong base produces a ______________ salt. The salt produced by the reaction of a weak acid with a weak base can be either acidic or basic depending on the Ka and Kb of the resulting ions. 2 4. Calculate the pH of a 0.25 M solution of ammonium chloride (this is nothing new). 5. Calculate the pH of a 0.25 M solution of sodium acetate (this is nothing new). 3 Polyprotic acids Some acids have more than one ionizable proton. For example, sulfuric acid (H2SO4) is diprotic and phosphoric acid (H3PO4) is triprotic. 1. Write the equations for the dissociation of each proton in phosphoric acid. H3PO4(aq) + H2O(l) = Ka1 = 7.5 x 10-3 Ka2 = 6.2 x 10-8 Ka3 = 4.8 x 10-13 2. Why do you think Ka1 >> Ka2 >> Ka3? Note: For solutions of most weak polyprotic acids, you can get a very good estimate of the pH by using Ka1 only (The subsequent ionizations contribute very little to the hydrogen ion concentration.) 3. Write the base-dissociation equations for each of the 3 conjugate bases and calculate each Kb. Kb1 = Kb2 = Kb3 = 4 4. Predict whether each of the following solutions is neutral, acidic, or basic: NaH2PO4(aq) Na2HPO4(aq) Na3PO4(aq) 5