Essential Information Required for Changes to/or New Module
advertisement
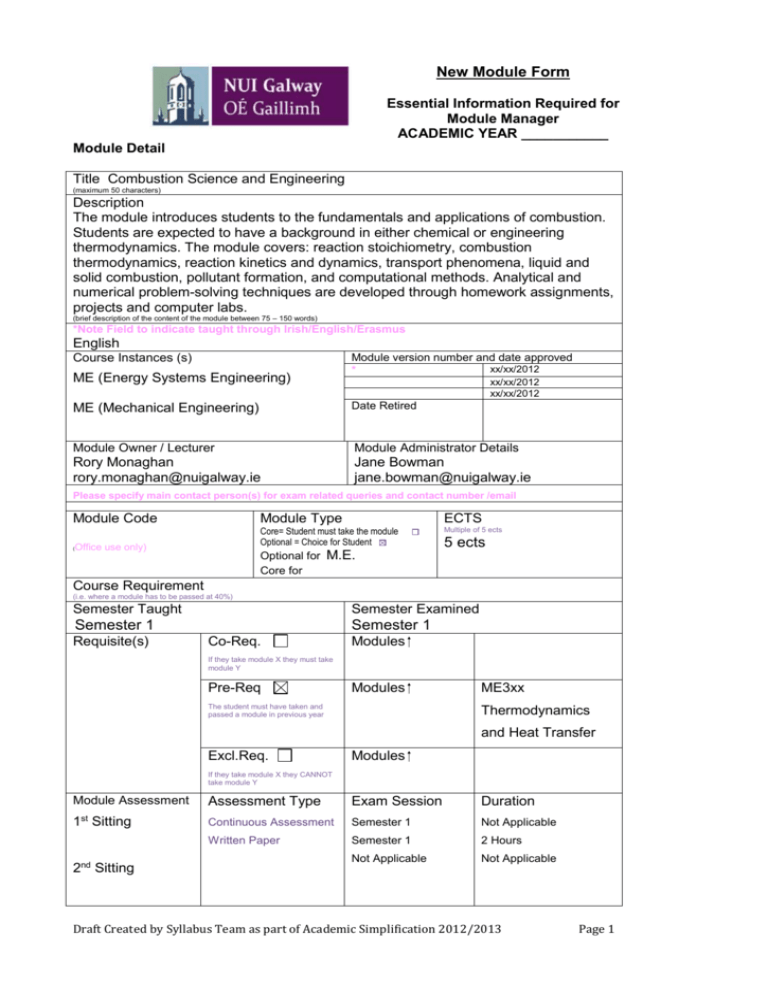
New Module Form Essential Information Required for Module Manager ACADEMIC YEAR ___________ Module Detail Title Combustion Science and Engineering (maximum 50 characters) Description The module introduces students to the fundamentals and applications of combustion. Students are expected to have a background in either chemical or engineering thermodynamics. The module covers: reaction stoichiometry, combustion thermodynamics, reaction kinetics and dynamics, transport phenomena, liquid and solid combustion, pollutant formation, and computational methods. Analytical and numerical problem-solving techniques are developed through homework assignments, projects and computer labs. (brief description of the content of the module between 75 – 150 words) *Note Field to indicate taught through Irish/English/Erasmus English Module version number and date approved xx/xx/2012 * Course Instances (s) ME (Energy Systems Engineering) xx/xx/2012 xx/xx/2012 ME (Mechanical Engineering) Date Retired Module Owner / Lecturer Module Administrator Details Rory Monaghan rory.monaghan@nuigalway.ie Jane Bowman jane.bowman@nuigalway.ie Please specify main contact person(s) for exam related queries and contact number /email Module Code ( Module Type Core= Student must take the module Optional = Choice for Student Office use only) Optional for Core for ECTS Multiple of 5 ects 5 ects M.E. Course Requirement (i.e. where a module has to be passed at 40%) Semester Taught Semester Examined Semester 1 Requisite(s) Semester 1 Co-Req. Modules If they take module X they must take module Y Pre-Req Modules The student must have taken and passed a module in previous year ME3xx Thermodynamics and Heat Transfer Excl.Req. Modules If they take module X they CANNOT take module Y Module Assessment st 1 Sitting 2nd Sitting Assessment Type Exam Session Duration Continuous Assessment Semester 1 Not Applicable Written Paper Semester 1 2 Hours Not Applicable Not Applicable Draft Created by Syllabus Team as part of Academic Simplification 2012/2013 Page 1 Bonded Modules (modules which are to be examined at the same date and time) Draft Created by Syllabus Team as part of Academic Simplification 2012/2013 Page 2 PART B Workload: ECTS credits represent the student workload for the programme of study, i.e. the total time the student spends engaged in learning activities. This includes formal teaching, homework, self-directed study and assessment. Modules are assigned credits that are whole number multiples of 5. One credit is equivalent to 20-25 hours of work. An undergraduate year’s work of 60 credits is equivalent to 1200 to 1500 hours or 40 to 50 hours of work per week for two 15 week semesters (12 weeks of teaching, 3 weeks study and formal examinations). Module Schedule No. of Lectures Hours 36 No. of Tutorials Hours 8 No. of Labs Hours 8 Recommended No. of self study hours 58 Other educational activities(Describe) and hours allocated Lecture Duration Tutorial Duration Lab Duration Placement(s) hours 0 0 1 1 2 *Total range of hours to be automatically totalled (min amount to be hit) Module Learning Outcomes (CAN BE EXPANDED) On successful completion of this module the learner should be able to: 1 Perform calculations of combustion stoichiometry 2 Use the 1st law of thermodynamics to calculate fuel heating value 3 Use the 2nd law of thermodynamics to calculate equilibrium compositions and flame temperatures for combustion 4 Calculate the chemical kinetic rates of combustion reactions 5 Use detailed chemical mechanisms to predict combustion characteristics of fuels under realistic conditions 6 Solve mixed diffusion-kinetic controlled combustion problems 7 Understand the interaction between fluid dynamics and chemical reactions 8 Solve combustion problems for liquid and solid fuels 9 Evaluate efficiency and environmental performance of energy conversion technologies Module Learning, Coursework and Assessment Learning Outcomes at module level should be capable of being assessed. Please indicate assessment methods and the outcomes they will assess Assessment type, eg. End of year exam, group project Continuous Assessment Written Paper Outcomes assessed % weighting 1-9 50 1-9 50 Indicative Content (Marketing Description and content) The module introduces students to the fundamentals and applications of combustion. Students are expected to have a background in either chemical or engineering thermodynamics. The module covers: reaction stoichiometry, combustion thermodynamics, reaction kinetics and dynamics, transport phenomena, liquid and solid combustion, pollutant formation, and computational methods. Analytical and numerical problem-solving techniques are developed through homework assignments, projects and computer labs. Draft Created by Syllabus Team as part of Academic Simplification 2012/2013 Page 3 Module Resources Suggested Reading Lists Library Physical (e.g. AV’s) "An Introduction to Combustion: Concepts and Applications" by Stephen R. Turns Journal IT (e.g. software + version) Cantera/Chemkin + ANSYS Admin FOR COLLEGE USE ONLY Student Quota Quota (where applicable only) (identify number per module where applicable only) Module: Number: Discipline involved in Teaching Share of FTE *(drop down for disciplines within school) Mechanical Engineering Chemistry *(% out of 1) 75 25 RGAM NB: Notes on some fields are for the technical side when considering which software company to use. Draft Created by Syllabus Team as part of Academic Simplification 2012/2013 Page 4