File - notes
advertisement

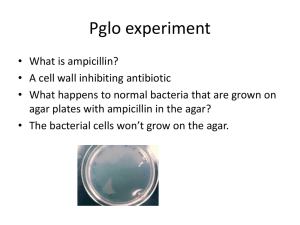
DNA Technology DNA Cloning Recombinant DNA – DNA that comes from two different sources Genetic engineering – the direct manipulation of genes for practical purposes Biotechnology – the manipulation of organisms to make useful products Steps to gene cloning o Isolate the gene of interest from a eukaryotic cell o Insert the gene of interest into a previously isolated bacterial plasmid o The recombinant plasmid is placed in a bacterial cell o Identify and clone recombinant bacteria o The gene itself or a gene product can be used for multiple things Confer pest resistance to plants Alter the metabolism of bacteria Produce human proteins Restriction enzymes – enzymes that cut DNA molecules at a limited number of specific locations o Produce “sticky ends” (single stranded ends) o DNA ligase reseals the ends of the recombinant DNA o Naming The letters refer to the organism from which the enzyme was isolated First letter – stands from the genus of the organism Second & third letters – species name Fourth letter (optional sometimes) – strain of the organism Roman numeral – whether the enzyme was first isolated, the second, etc. from the species o E = genus Escherichia co = species coli R = strain RY13 I = first RE isolated from this species Recognition sites – sequences of DNA that restriction enzymes recognize in order to cut the DNA Cloning vector – a DNA molecule that can carry foreign DNA into a cell and replicate there o Plasmid (bacteria & certain fungi) o YAC (yeast artificial chromosome) o Virus o EcoRI Nucleic acid hybridization – process used to identify gene of interest o Radioactive ssRNA or DNA is used o Complementary sequence hydrogen binds to the gene of interest o Requires some knowledge of the sequence o Requires denaturation of DNA strands of the plasmid Expression vectors carrying eukaryotic genes often have a prokaryotic promoter upstream of the gene in order for RNA polymerase to attach and transcribe the foreign DNA cDNA o DNA that was synthesized from the reverse transcription of mRNA o Lacks eukaryotic introns Yeast (eukaryote!) contains plasmids Electroporation o Applying a brief electrical pulse to a solution containing cells o Done in order to create temporary holes in the plasma membrane for foreign DNA to enter RFLP’s (restriction fragment length polymorphisms) o Restriction enzymes are used to cut up different individuals’ genomes in order to compare them PCR (polymerase chain reaction) o Quickly amplifies any piece of DNA without using cells Target sequence production increases exponentially o Replicates only target sequences (not whole genome) o Steps Stage 1 (Denaturing) Heat briefly to separate DNA strands ~ 95°C Stage 2 (Annealing) Cool to allow primers to hydrogen-bond to DNA 50-65°C Stage 3 (Extending) DNA polymerase adds nucleotides to the 3’ end of each primer ~ 72°C o Primers must be present in excess; increases likelihood of attachment to DNA o Overshoot strands – strands that contain DNA outside the target sequence o Taq polymerase used in PCR Comes from bacteria found in hot springs Can withstand high temperatures Won’t denature during the denaturing process o Two primers are necessary per target sequence (one for each DNA strand) o Overshoot DNA fragments are often not visible if run through gel electrophoresis with the other target sequence fragments because there are not that many when compared to the number of target sequence fragments Similarities between PCR and RFLP o Use gel electrophoresis o Produce fragments o Use satellite regions (regions of repetitive DNA) Differ between individuals in the number of repetitive sequences Differences between PCR and RFLP o PCR – uses only target sequence and primers, doesn’t cut DNA o RFLP – uses entire genome and restriction enzymes, cuts DNA DNA Analysis and Genomics Genomics – the study of whole sets of genes and their interactions Gel electrophoresis – sorts nucleic acids and proteins based on size, electrical charge, and other physical properties o Gel used for electrophoresis is a matrix and contains pores that DNA fragments can squeeze through o The smaller the DNA fragment, the faster it travels through the gel and the further down the gel it appears o DNA marker contains fragments of known length; a marker must be used with each test to estimate the length of the fragments run through the gel Southern blotting combines gel electrophoresis and nucleic acid hybridization to reveal: o Whether a particular sequence is present in a sample of DNA o The size of the restriction fragments that contain the sequence Human Genome Project (HGP) o Goal: map the entire human genome o Stages: Genetic (linkage) mapping The relative distances between gene markers can be determined by recombination frequencies Physical mapping The distances between markers are expressed in some physical measure, typically the number of nucleotides along the DNA A common method for this is known as “chromosome walking” DNA sequencing The Sanger method uses DNA labeling, DNA synthesis that used special chainterminating nucleotides and high resolution gel electrophoresis Microarray analysis – used to determine which genes are expressed in particular tissues In vitro mutagenesis – specific mutation are introduced into the sequence of a cloned gene and the gene is returned to a cell; the phenotype of the mutant cell may help reveal the function of the missing normal protein SNP’s (single nucleotide polymorphisms) o Single base-pair variations in the genome o Occur on average about once in 1,000 base pairs o Humans are 99.9% related to each other Prokaryotes – most DNA codes for protein Eukaryotes - ~97% does not code for protein (nor rRNA, tRNA) o Regulatory sequences Promoter, terminator, enhancers, control elements o Introns Code for snRNPS o Repetitive DNA Positive: Telomeres Centromeres Negative Fragile X Huntington’s disease o ncRNA miRNA siRNA Gene amplification o Repetitive sequences of genes increase the genes expression o Transposition may result in amplification or loss of some genes Could increase or decrease transcription Retrotransposons o Transcribed RNA includes the code for an enzyme that catalyzes the insertion of the retrotransposon (using reverse transcriptase) Practical applications of DNA technology o Gene therapy Ex) a normal allele is inserted into somatic cells of a tissue affected by a genetic disorder; alleles has to multiply throughout the patient’s life in order to be permanent (not very effective) o Pharmaceutical use Ex) hormones, vaccines o Forensics o Genetically modified organisms (GMOs) Transformation Lab Goals: o To transform and isolate E.coli bacteria with a previously engineered bacterial plasmid Note: the bacterial plasmid has been engineered to contain a eukaryotic gene using the same process as modeled in the paper plasmid lab o To identify positive and negative controls designed within the experiment o To express the eukaryotic gene in the prokaryotic cell o To consider the practical applications of bacterial transformation in everyday life The bacterium: E.coli: o This is a non-pathogenic strain of the bacteria; however, the lab design includes steps to ensure aseptic technique and to treat the bacterium as if it could cause harm o The original bacterial colonies used do not have resistance to ampicillin The pGlo plasmid: o AmpR Gene: This is a gene already within the plasmid that confers resistance to the antibiotic ampicillin (This not the eukaryotic gene that was introduced) The gene codes for the enzymes beta-lactamase that preventsampicillin from entering a bacterial cell. Thus, if the bacterial cell contains this plasmid, it will have the AmpR gene and it will be able to survive in the presence of ampicilin. o Green Fluorescent Protein (GFP) Gene: This is a gene isolated from a deep sea jellyfish (a eukaryote). The gene codes for a green fluorescent protein (this protein fluoresces in black light). The gene has been cut out form the jellyfish DNA using restriction enzymes and inserted within the arabinose operon on the pGlo plasmid. o Arabinose Operon: This is an operon found within the pGlo plasmid The operon normally functions to code for proteins that will breakdown the sugar arabinose The plasmid has been engineered to contain the gene for GFP within the arabinose operon. Thus, when the operon is turned on (in the presence of arabinose sugar) the GFP gene will also be expressed and GFP will be produced Basic procedure o You are going to take 2 bacterial colonies. -pGlo bacteria: These bacteria will NOT be exposed to the plasmid for transformation +pGlo bacteria: These bacteria will be exposed to the plasmid for transformation o For both you will heat shock the bacteria The heat makes the membrane more porous and able to accept DNA from the environment (the plasmid) This procedure is NOT very efficient; not all bacteria will be transformed But, out of millions of bacteria some should be transformed o For both, you will add calcium ions. The positively charged calcium ions coat the negatively charged bacterial cell membrane and pull the negatively charged DNA closer to the cell membrane o You will introduce the bacteria into 4 specific agar plates LB-pGlo: This plate contains only Luria Broth and is not exposed to the plasmid LB/Amp-pGlo: This plate contains both the Luria Broth nutrients and the antibiotic ampicillin, and is not exposed to the plasmid LB/Amp+pGlo: This plate contains both the Luria Broth nutrients and the antibiotic ampicillin, and is exposed to the plasmid LB/Amp/Ara+pGlo: This plate contains Luria Broth nutrients, the antibiotic ampicillin, the sugar arabinose and is exposed to the plasmid Predictions for the plates: o LB-pGlo: Lawn of bacteria No ampicillin to kill the bacteria o LB/Amp+pGlo: No arabinose to turn on the Some bacteria operon Ampicillin-treated plate No glowing because the operon Some bacteria absorb the plasmid is not turned on No arabinose to turn on the operon o LB/Amp-pGlo: No glowing because operon is not turned on No bacteria o LB/Amp/Ara+pGlo: Ampicillin-treated plate Some bacteria No plasmid so no ampicillin Ampicillin-treated plate resistance Some bacteria absorb the plasmid No arabinose and no bacteria for Arabinose present – operon is turned on operon to turn on Glowing plate because operon is on No glowing because the operon is not turned on/present Controls o Experimental plate(s): This plate shows the effect of our experimental treatment on the bacteria LB/Amp+pGlo LB/Amp/Ara+pGlo o Gene Expression Plate: This plate shows evidence of our manipulation of gene expression. It shows if we have expressed the gene successfully LB/Amp/Ara+pGlo o Positive Control: This plate is used to see what bacterial growth should look like before applying our experimental treatment/variable. If there is no bacterial growth, it indicates that some error has occurred in the lab and the consequent results may not be reliable LB-pGlo o Negative Control: This plate is used to see what no bacterial growth should look like before applying our experimental treatment/variable. If there is bacterial growth, it indicates that some error has occurred in the lab and the consequent results may not be reliable LB/Amp-pGLO Transformation efficiency Total number of colonies growing on the agar plate o Transformation efficiency = Amount of DNA spread on the agar plate (in μg) o o Calculate the total number of transformed cells Count the number of colonies on the LB/Amp/Ara+pGlo plate Calculate the amount of plasmid DNA in the bacterial cells spread on the LB/Amp/Ara+pGlo plate Calculate the total amount (mass) of plasmid DNA DNA in µg = (concentration of DNA in µg/µL) x (volume of DNA in µL) Calculate the fraction of plasmid DNA that actually got spread onto the LB/Amp/Ara+pGlo plate Volume spread on the LB/Amp/Ara+pGlo plate (μL) Fraction of DNA used = Total sample volume in test tube (μL) o Calculate the micrograms of plasmid DNA that you spread on the LB/Amp/Ara+pGlo plate DNA spread in µg = (total amount of DNA used in µg) x (fraction of DNA used) Calculate transformation efficiency using formula