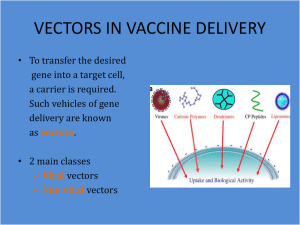
In vivo viral gene delivery
advertisement

SOP In vivo viral gene delivery Starr Lab PI: Timothy K. Starr Lab Location: 12-175 Moos Towers Original issue date: 11/29/11 Revision dates: 3/4/14 Prepared by: Timothy K. Starr and Lee Pribyl Approved by: Timothy K. Starr Table of Contents Table of Contents ................................................................................................................................................ 1 Overview ................................................................................................................................................................ 1 Background........................................................................................................................................................... 1 Antimetabolites ........................................................................................................... Error! Bookmark not defined. Plant derivatives ......................................................................................................... Error! Bookmark not defined. Alkylating agents......................................................................................................... Error! Bookmark not defined. Targeted chemotherapeutics ................................................................................. Error! Bookmark not defined. Procedures ............................................................................................................................................................ 1 General safety procedures........................................................................................................................................... 3 Preparation of chemotherapies................................................................................................................................. 3 Dosing of chemotherapies ....................................................................................... Error! Bookmark not defined. Intra-peritoneal (IP) injection of chemotherapies ............................................................................................. 5 Intra-venous (IV) injection of chemotherapies in tail vein ......................... Error! Bookmark not defined. Oral gavage administration of chemotherapies .............................................. Error! Bookmark not defined. Hazard Identification and Risk of Exposure to the Hazards: .............................................................. 5 Exposure Controls Specific to Above Risk of Exposure: .................................................................................... 8 Waste Generated and Disposal Methods .................................................................................................... 8 Spill and Accident Response Procedures ................................................................................................... 9 Further sources of information ..................................................................................................................... 9 References ......................................................................................................... Error! Bookmark not defined. Overview This protocol covers procedures related to delivery of viral vectors to mice Background The following background material on in vivo viral delivery was adapted from information provided by the University of Texas Health Science Center at Houston, Office of Research: http://www.uth.tmc.edu/safety/biosafety/adenovirus.htm General health Information on Human Adenovirus There are approximately 50 different serotypes of human adenoviruses. Some can induce a spectrum of illnesses including acute, self-limiting pharyngitis (a common cold), keratoconjunctivitis (pink eye), and diarrhea. In rare cases, human adenovirus may cause hepatitis (inflammation of the liver), or inflammation of other organs. Also, in rare situations when an individual is already seriously immunocompromised (has weakened defenses against infections, i.e. AIDS patients), the outcome could be a blood infection. However, in most instances, adenoviruses cause only a common upper respiratory illness known as a "cold." One of the most common adenovirus serotypes that infect the human respiratory tract is Adenovirus Type 5. This is the serotype that recombinant adenoviral vectors are currently derived from. The epidemiology of adenovirus indicates that it is found worldwide with high occurrences in children under the age of 5 years. In tropical regions, adenovirus incidences usually occur in wetter areas, while temperate regions experience seasonal occurrences with the highest incidences occurring in the fall, winter, and early spring. The mode of transmission of adenovirus is directly by oral contact or aerosolized droplet exposure to the mucous membrane with an incubation period of 1 to 10 days in healthy humans. There are no specific antiviral drugs currently available, but adenovirus shows susceptibility to chemical disinfectants such as 1% sodium hypochlorite and 2% glutaraldehyde. A question is occasionally raised by individuals with regard to the potential problem of an investigator suffering from a cold while working with adenoviral vectors. Most adult individuals have had natural adenoviral infection early in childhood, which results in immunity to subsequent adenoviral infections. If Biosafety Level 2 precautions are strictly followed, there is a very low risk of self-contamination with the vector. Recombinant Adenoviral Vectors Laboratory-made recombinant adenoviral vectors are derived from the type 5 adenovirus. Common deletions in adenoviral vectors include the E1 and E3 regions. The E1 deletion renders the virus incapable of autonomously reproducing itself and the E3 deletion makes the virus more susceptible to the human immune defense system and also provides an area for transgene insertion. The E1 deletion is replaced with an "expression cassette" that consists of a promoter, research gene and poly A signal. The recombinant vector can be produced to very high titers in Human Embryonic Kidney (HEK) 293 cells (the titer can reach up to 10^12 infection units per millimeter). There are several points that must be kept in mind with regard to recombinant vectors: Despite the fact that humans are probably immune to this vector, a vector may still infect an individual if he/she is exposed to a high titer. Nonetheless, theoretically, recombinant adenoviral vectors would not replicate. While exposure to wild type, replication competent adenovirus may be a low risk, the risk of exposure to recombinant adenoviral vectors is unknown. Moreover, the potential risk of exposure to different recombinant adenoviral vectors may not be the same. It is believed that some vectors may have minimum risk (e.g., null and LacZ vectors) while others (e.g., interleukin, TNF, or immune effector vectors) may pose a higher risk. Based on the current understanding, recombinant adenoviral vectors have been classified as Class I (minimum risk) and Class II (potentially higher risk). The safety conditions under which these two classes are to be used are very similar, that is, generally all procedures are performed under Biosafety Level 2 (BSL-2), while the Class II vectors are performed under Biosafety Level 2 with the possible addition of Biosafety Level 3 practices and/or equipment. The classification of adenoviral vectors into classes is only intended as a guide. Based on clinical data, the wildtype virus (adenovirus type 5) was categorized in the Class I classification. All other categorizations are less clear. The attenuated viruses with the E1 region deletions might naturally be expected to be less dangerous, due to their reduced capacity for autonomous viral replication, and categorized as Class I. Based on transgenic animal studies, vectors expressing transgenes such as markers (e.g., LacZ, neomycin phosphotransferase, and chloramphenicol acetyl transferase) are grouped in Class I. On the other hand, vectors expressing a product that is known to be toxic or involved in the regulation of cell growth should be grouped in Class II. As alluded from the above, if the recombinant vector being studied has minimal safety data, or there is concern over the over-expression of the transgene(s) or the location of the transgene(s) expressed, a conservative safety approach should be taken and the use of Biosafety Level 2+ practices should be used when appropriate. Procedures General safety procedures All handling and injection of virus is to be performed in a Biological Safety Cabinet (BSC) using BSL2 practices. Two BSC hoods will be used, one in MoosT 12-175, and one in MCB 1-134A. Wear Personal Protective Equipment (PPE): sterile gloves, eye goggles, lab coat and arm sleeves. Gloves must be worn at all times when working with virus. Remove gloves using the inside-out technique. Dispose of gloves into biohazard waste container to be autoclaved. Wash hands immediately after removing gloves and before leaving work area. Never wear gloves outside of the laboratory, or touch doorknobs, telephones, personal belongings, etc. with gloved hands. Transport virus by foot in a sealed, leak-proof primary container within a sealed, leak-proof secondary container with sufficient absorbent material between the two containers (e.g. Screw cap tube inside a 15mL conical tube). Attach a biohazard label to the outside listing: name, contact address, phone #, name of reagent, emergency contact. The most effective germicide against virus includes 30-minute exposure with 10% bleach (1:9 v/v) solution. Before working the BSC If working in MCB 1-134E (animal facilities) post a Biohazard sign on door indicating that unauthorized personnel may not enter during the experiment. Remove all unnecessary equipment and supplies from the BSC in order to ensure smooth air flow. Check that air grilles are clear. Turn on blower before using to remove particulates in the cabinet. Wait at least five minutes. Wipe down surface of cabinet interior with disinfectant (paper towels soaked with 10% bleach followed by spraying with 70% ethanol). Place supplies and needed equipment in the BSC before beginning work to minimize the number of arm-movement disruptions across the air barrier of the cabinet. Only items required for the immediate work should be placed in the BSC. Place absorbent towels and decontaminating solution (70% ethanol) near the BSC to facilitate quick clean up of spills. Wipe the exterior of supplies with a disinfectant (70% ethanol), particularly containers removed from a water bath. Spray items with a fine mist of 70% ethanol, this will evaporate quickly and leave no wet mess but still be effective. Segregate items that will remain clean from the ones that may become contaminated. Wash hands and arms, wear appropriate protective equipment for the work being done and to prevent skin flora from contaminating your work. Adjust stool height so that your neck and face are above the sash opening. While working in BSC Delay manipulation of materials for approximately one minute after placing the hands/arms inside the BSC. Do not rest arms on the front grille. Raising arms slightly will lessen disruption of air flow. Work as far back in the cabinet as practical - at least four inches inside the front grill edge. Move arms slowly and limit arm movement in and out of cabinet. As a general rule of thumb, keep clean materials at least one foot away from aerosol-generating activities to minimize the potential for cross-contamination. The work-flow should be from "clean (left) to contaminated or dirty (right) ". Limit the movement of "dirty" items over "clean" ones. Remove media with vacuum and replace with serological pipettes. After working in BSC: Wipe down the surfaces of all containers and equipment with an appropriate disinfectant (70% ethanol) and remove from the BSC. Wipe down the cabinet interior with disinfectant (paper towels soaked with 10% bleach followed by spraying with 70% ethanol. Leave blower on for several minutes with no activity so that any airborne contaminants will be purged from the work area. Remove gloves and dispose in Biological Hazards bag and wash hands. Tips to prevent contamination Clean water baths frequently and/or treat water in bath. Clean the inside of incubators frequently, particularly the water tray. Use treated di water in the bottom of the incubator or water tray. Use HEPA filters on incubator CO2 and air intake lines. Replace regularly. Lab coat sleeves can introduce contaminants to biological safety cabinets and incubators. Use coats designated for working in the biological safety cabinet or tissue culture area, launder frequently. Use disposable sleeve guards if contamination has been a problem. Never pour media, remove with vacuum and replace with disposable pipettes. Do not leave flasks of waste media in cabinet, clean after every use. On a regular basis, decontaminate under the air grilles and wherever parts are removable. Media is commonly splattered on the front grille allowing fungus to grow undetected on the under surface of the grille. Decontaminate the surface of carts or trays used to transfer culture flasks between the incubator and the biological safety cabinet or microscope. Keep pipette aids cleaned, especially the nosepiece, and replace filters regularly. Clean and disinfect vacuum tubing. This should be done by sucking up the disinfectant we place in the bottom of the waste flask after we have emptied it and reattached it. Keep the water in the incubator's water jacket full. If water levels in the jacket drop, the ceiling of the incubator will be cooler causing condensate to form. Water then drops onto shelves and cell culture containers. Check port plugs and septums for contamination in incubator interior; they may trap moisture and harbor fungi. Materials Mice in SPF microisolator cages Sterile stock solutions of anesthetizing reagents: ketamine hydrochloride (100 mg/ml) xylazine hydrochloride (100 mg/ml) acepromazine (10 mg/ml) saline solution (0.9% NaCl) Cre-Recombinase Adenovirus. Titer of virus is generally between 1 x 10^7 to 1 x 10^10 PFU/ml (1 x 10^4 to 1 x 10^7 PFU/µl). Virus is purchased from a commercial source such as Eton Biosciences (Cat # 0100062001) or Cell BioLabs (Cat # ADV-005). The virus is NOT replication competent and contains the Cre Recombinase gene. Ketoprofen (brand name ketofen). Bottle of 70% ethanol 23g needles and 1 ml syringes for injection of anesthesia 30g needles and 1 ml syringes for injection of virus Sterile pads and gauze Procedure Follow procedure for using BSC listed above. Place all materials in BSC, including mouse. Weigh mouse Prepare Anesthesia cocktail. For a 25 g mouse a single dose of 200 µl is prepared by drawing up the following amounts from the original bottles and combining in a single syringe: 7.5 µl of 100 mg/ml ketamine (= 30 mg/kg) 1.5 µl of 100 mg/ml xylazine (= 6 mg/kg) 1.25 µl of 10 mg/ml Aceprozamine (= 0.5 mg/kg) 189.75 µl of 0.9% saline solution Use the Anesthetic dosing Xcel spreadsheet to calculate amount needed for entire experiment. For the secondary anesthesia, if required, omit the xylazine and aceprozamine and increase the saline to 192.5 µl. Load 23G syringe with appropriate volume of 1º anesthetizing reagent. Load 30G syringe with 10 µl of virus (between 1 to 10 x 10^5 PFUs). Anesthetics procedure Firmly hold mouse by scruff of neck pinched between forefinger and thumb, with tail tucked under little finger. Insert needle about 10 mm ventrally from rear leg, taking care to avoid nipples and inject 1º anesthetizing reagent into the intraperitoneal cavity. Wait until mouse is sufficiently sedated. Mouse should not have any reflex when toe is pinched. If surgery lasts longer than 20 minutes, make a second injection using the 2º anesthetizing reagent (ketamine and acepromazine only). Surgery Lay animal dorsal side up on sterile gauze with head facing away, tail towards you Shave or wet fur with 70% alcohol at incision point. Lift wetted skin using forceps and make small incision in the skin (not the peritoneal wall) with scissors at dorsomedial position and directly above the ovarian fat pad. The fat pad should be visible beneath surface of peritoneal wall. Fat pad is recognizable by its white color in contrast to the dark pink tissue surrounding it. Make a second incision in the peritoneal wall just above the fat pad. Place a sterile soaked saline gauze pad on the midline adjacent to the incision. Locate ovarian fat pad and pull it out and rest it on the gauze. Stabilize the ovary by clamping fat pad with a bulldog clip. Under a dissecting microscope, position ovary as to allow for insertion of a 30 G needle into the oviduct tubule bend leading to the bursa. The needle should be inserted close enough to the infiundibulum to see it under the bursa and the ovary. When needle is inserted into proper position, it should be visible under the bursa. Gently push plunger of syringe to inject between the bursa and the ovary while the syringe is position at the injection site. This works best with two people. One person holds the needle in place while the second person pushes the plunger. A maximum of 10 – 20 µl can be injected. Remove the needle quickly to seal puncture site, but gently enough not to tear the bursa and tubule. Bursa should appear to be slightly distended with proper injection. Release fat pad from bulldog clip and gently replace ovaries into peritoneal cavity using forceps. Gently close body wall by pulling the upper peritoneal lining over the lower lining. Optional to close incision in peritoneal wall with 2 – 4 sutures using 6-0 silk or Vicryl sutures. Close skin with surgical staples or wound clips. Place animal back in cage with a heat source to avoid hypothermia and speed up recovery. Post Surgery Immediately following surgery mice will be given a subcutaneous injection of Ketoprofen to reduce pain. We will use a dose of between 2 to 10 mg/kg, starting with 5 mg/kg. Mice will be monitored daily for wellness, palpable tumors and any procedure-related complications such as organ failure, thrombosis or ischemia. Incision will be monitored for swelling, exudate, pain or dehiscence. Antibiotics will be administered if there are post-procedural infections, according to RAR procedures. Ibuprofen will be administered if there are signs of pain. Administration will be 10 ml Children’s Motrin in 500 ml drinking water and maintained daily until signs of pain are gone. Remove staples or wound clips 7 or more days post surgery. Sacrifice mice when moribund, at tumor endpoint, or at 18 months. See original protocol for detailed description of endpoints and signs of distress. Post Surgery Housing The IBC has determined that mice inoculated with replication-incompetent adenovirus expressing Cre Recombinase may be returned to ABSL-1 rodent housing rooms if they are housed in cages with secured filtertops for a three-day 'quarantine' period following inoculation. After inoculation the site of inoculation on the animal will be thoroughly cleaned to remove residual vector Mice are then placed in clean filter-topped cages while still in the biosafety cabinet using BSL-2 procedures. The cage filtertops will be secured to the clean cages with tape or rubber bands. A card will be placed on each cage specifying: date at the end of the three day quarantine period only the Investigator's staff may open the cage prior to that date identity of the vector 24 hour contact information so that the animal care staff can reach the Investigator's staff in case a cage needs to be opened. The cages are then returned to the ABSL-1 housing room using secondary containment during transport. Any cages which need to be opened during the quarantine period will be opened only by our staff, in biosafety cabinets using BSL-2 procedures. If our staff cannot be reached in an emergency, trained RAR staff will open cages in biosafety cabinets, using BSL-2 procedures. The card is removed from the cage at the end of the quarantine period. Routine animal care resumes after the quarantine period. Peri-operative Record Keeping For all surgical and anesthetic procedures, a real time procedural record will be kept and maintained as part of the animal’s records. Records will be maintained for three years to cover the life of the protocol. Records will include: Animal or group identification, protocol number, and the date of the procedure (identical surgeries on multiple animals will be listed in one record), All drugs or fluids administered, including dose, route, time, and the identity of the person administering the drugs, A description of the surgical procedure and identification of the surgeon(s), Ongoing findings during monitoring (eg. Heart rate, depth of anesthesia). For surgeries on more than one animal conducted during the same surgical setup period, findings may be noted after the end of the surgery. Notation of any variations from the normal and expected events during the anesthetic and recovery periods, including the actions taken and the time performed, the animal’s response to these actions, and the identity of the person performing these actions, Assessment for pain and distress, Actions taken to alleviate pain and distress, including non-pharmacologic interventions, and the response to these actions, A notation defining the end of the monitoring period (euthanasia or functional recovery from the sedation or anesthesia), including the time, date, and the identity of the person making the notation. Post-Surgical Monitoring Records Post operative records will include the time of monitoring, any observed problems, what type of analgesics/antibiotics are given, if any (including dosages and route), whether the animals are active, eating, drinking and whether the incision site is healing well or is infected, animal posture, fur coat texture (indicators of pain), urine and feces production. These records will be kept for at least 3 days post-surgically for minor surgical procedures or if the surgery is more invasive, until the post operative period is at an end (for example, when sutures are removed and surgical wounds are adequately healed). All records will be readily available to the personnel involved in post surgical monitoring, the veterinary staff, the IACUC, and federal regulatory officials Hazard Identification and Risk of Exposure to the Hazards Two main hazards exist: Sharps and possible exposure to viral vectors expressing oncogenes and protooncogenes. Note: this is especially hazardous for immunosuppressed or pregnant laboratory staff. Any of these symptoms may occur following adenovirus exposure: Acute respiratory illness (cold like symptoms) Pneumonia Conjunctival infection (red eye) Corneal inflammation leading up to scarification Exposure Controls Specific to Above Risk of Exposure: PPE - Lab coats, safety goggles, gloves, surgical mask, sleeves and sharps containers. Use Biologic Safety Cabinet during preparation and handling of virus All sharps and glass waste will be disposed of in an approved hard plastic sharps container (U Stores # CX40245, MS07407 or similar), No Cardboard sharps pouches. If exposure to virus occurs consult a physician. Show the MSDS to the doctor in attendance. Move out of dangerous area. If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician. In case of skin contact wash off with soap and plenty of water. Consult a physician. In case of eye contact flush eyes with water as a precaution. If swallowed never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. In case of accidental injection seek medical attention immediately. In general, decontamination is done using bleach. Keep near the hood a bottle of freshly made 10% bleach (1:9 v/v) solution and a 70% ethanol spray bottle and a bottle of dH2O for rinsing Decontamination of liquid waste (conditioned medium and virus containing samples) should be performed in final 10% bleach (1:9 v/v) solution for 30 minutes. It may then be sewered followed by copious amounts of water. Wear a face protection shield (prevents splashes to your face while decontaminating). Waste Generated and Disposal Methods Liquid waste will be collected in a flask or beaker containing bleach 10% (v/v) and will soak for 30 minutes before being sewered. Solid waste decontamination Each used pipette, plate, dish, tube and tip has to be washed with 10% bleach (1:9 v/v) solution before discarding it to the biohazard bag. Alternatively you can dip the used pipette in a box containing 10% bleach (1:9 v/v) solution (for example sharps box) provided that enough volume will be used to cover 20% of pipette height. Used tips should be soaked in a plastic bottle (for example, cleaned used medium bottle) containing 10% bleach (1:9 v/v) solution. Used plates/flasks have to be decontaminated with 10% bleach (1:9 v/v) solution for 30 minutes. Discard all decontaminated solid waste in the biohazard bag for incineration. Sharps containers will be sealed when ¾ full and placed in designated waste area. Refer to the Biological Waste Disposal procedures posted on the Tissue Culture room door for more information Spill Response Procedures For spill, splash or aerosol clean up: In general, decontamination is done using bleach. Keep near the hood a bottle of freshly made 10% bleach (1:9 v/v) solution and a 70% ethanol spray bottle and a bottle of dH2O for rinsing Decontamination of liquid waste (conditioned medium and virus containing samples) should be performed in final 10% bleach (1:9 v/v) solution for 30 minutes. It may then be sewered followed by copious amounts of water. Decontaminating a small volume spill, large volume spill and any splashes Wear a face protection shield (prevents splashes to your face while decontaminating), Cover the spill with paper towel and gently pour on top 10% bleach (1:9 v/v) solution for 30 minutes, followed by a rinse with water to remove remaining bleach that may pit or etch work surfaces & equipment if needed, then followed by a 70% ethanol rinse Collect the paper towels to the biohazard bag. Refer to the Biological Decontamination and Spill Clean-up Plan posted on the Tissue culture room door for more information Accident Response Procedures If Incident Results in a Hazard Exposure ( i.e. face or eye splash, cut or puncture with sharps, contact with non-intact skin): Encourage needle sticks and cuts to bleed, gently wash with soap and water for 5 minutes; flush splashes to the nose, mouth, or skin with water; and flush eyes at the nearest eyewash station with clean water for 15 minutes. Call 911 or seek medical attention. o For urgent care employees may go to HealthPartners Occupational and Environmental Medicine (M/F day time or Urgent Care after hours), or UMMC-Fairview Hospital (24 hrs). You may seek medical attention at the closest available medical facility or your own healthcare provider. o Follow-up must be done by HealthPartners Occupational and Environmental Medicine. Report the incident to your supervisor as soon as possible, fill out the appropriate documentation. o Employee First Report of Injury o Supervisor Incident Investigation Report Send Incident Report Form to the IBC if exposure has occurred during work on an IBC protocol. Report all biohazard exposures to the Office of Occupational Health and Safety (612-626-5008) or uohs@umn.edu. Note: It is important to fill out all of the appropriate documents to be eligible to collect workers compensation should any complications from the hazardous exposure arise in the future. References For further information view the UMN DEHS website containing Bio Basic Fact Sheets at http://www.dehs.umn.edu/bio_basicfacts.htm. For general information on Biosafety, access the Biosafety in Microbiological and Biomedical Laboratories (BMBL) 5th Edition from the CDC at BMBL http://www.cdc.gov/biosafety/publications/bmbl5/index.htm For Material Safety Data Sheets access the Public Health agency of Canada website MSDS http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/index-eng.php.