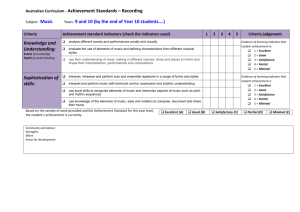
Self-Assessment:
Peer Review Criteria
Prior to doing the alterations (with respect to feedback) I would have said all these categories would
have needed some improvement but now I believe the report has mostly answered the questions
below. The results section was only satisfactory since the calculated equations aren’t given. For the
purposes of this item they weren’t calculated but cannot be accounted for.
Does the title clearly tell the reader what the article is
about?
Is the abstract sufficiently informative, especially when
read in isolation?
Is the article clearly written, free from spelling and
grammatical mistakes, and formatted correctly?
Is the organisation of the article satisfactory? Eg are
results only presented in the discussion section?
Is sufficient background provided in the introduction
and are the aims of the article clearly stated?
Is the description of the materials and methods
adequate to permit someone else to repeat the
study/experiment?
Do the experimental methods and data address the
aims and hypotheses?
Are the results clearly presented?
Does the evidence presented (from this or other
papers) support the ideas discussed?
Are topics discussed consistent with the aims,
background and hypotheses?
Are references provided to support all appropriate
claims or statements in the paper?
Absolutely
Absolutely
Absolutely
Absolutely
Absolutely
Absolutely
Most
ly
Most
ly
Most
ly
Most
ly
Most
ly
Most
ly
Satisfactory
Satisfactory
Satisfactory
Satisfactory
Satisfactory
Satisfactory
With improvement
With improvement
With improvement
With improvement
With improvement
With improvement
No
Absolutely
Absolutely
Absolutely
Absolutely
Absolutely
Most
ly
Most
ly
Most
ly
Most
ly
Most
ly
Satisfactory
Satisfactory
Satisfactory
Satisfactory
Satisfactory
With improvement
With improvement
With improvement
With improvement
With improvement
No
No
No
No
No
No
No
No
No
No
Reflection on changes:
With the alterations made I believe the report appeals to a wider audience and the chances of
misinterpretation have been reduced. Beforehand it may have been directed towards a specific
audience with assumed knowledge on the subject matter. As per the helpful feedback received I have
introduced a sentence to the ‘Abstract’ and restructured certain sentences to ensure the
communication is concise. I then deliberated with my own assessment making sure that the major notes
were implemented – IE labeling acronyms on first cite. After reading the final product it can be seen
that the feedback and self-assessment have provided valuable insight into the communication aspect of
the report. The process of using the information and translating it to the written text was simple but not
practiced prior. It becomes clear the importance of the principles we have discussed in class and their
effect on the appeal to audiences.
Determination of Glucose and
Sucrose in Sports Drinks
Communicating in Science
S4293404
S42934048
SCIE3001
1|Page
Abstract
Enzymes react sporadically, causing fluctuations and increases in cellular activity. They are used as a mechanism of control
for which cells can catalyse priority specific tasks. Through spectrophotometry it can be observed that the presence of
NADPH at certain time intervals can indirectly gauge enzymatic reactions occurring – additionally the products formed as a
result. Two sports drinks were assigned samples and tested for two types of carbohydrate – sucrose and glucose. The
enzyme reactions were coherent in respect to concentration levels. This experiment therefore tested for the presence of
either sucrose or glucose and their concentrations in sports drinks with respect to the nutritional labels given. The
manufacturer’s published label and the results of the experiment were indicative of accuracy.
Introduction
Enzyme induction is a process by which cells regulate protein synthesis via substrate mechanisms.
Activation energy is the required state before any reaction is initiated . The presence of enzymes
lowers the threshold thus liberating a lower energy pathway for the action potential to
propagate along. Enzymatic control does not impede/contribute to the reaction equilibrium and any
changes to reaction energy that may occur. Substrates are a counterpart to enzyme control which when
combined results in a product. In some cases, enzymes require the presence of another enzyme (coenzyme) or co-factor (for example the ions; Mg2+, Cu+ and Mn2+) to facilitate the reaction. (Timberlake,
2001).
Enzyme assays are used to measure enzymatic activity in reactions. By measuring either the
consumption of substrates or the amount of product being produced allows enzyme statistics to be
calculated. In this experiment, the continuous assay used involved light to determine the presence of
molecules – spectrophotometry. The type of molecule determines the wavelength’s absorbency. For
example, NADP+ (Nicotinamide adenine dinucleotide phosphate) cannot absorb strongly at 340nm
whereas NADPH (reduced Nicotinamide adenine dinucleotide phosphate) can. Therefore molecules that
disappear and reappear throughout the entire reaction can be measured spectrophotometrically. The
aim of the experiment is to test for the presences of these molecules and measure their concentrations
respectively.
S42934048
SCIE3001
2|Page
Reaction Sequence of the Experiment
Sucrose + H2O (water) à D-glucose + D-fructose
Enzyme: Invertase
D-glucose + ATP (Adenosine Triphosphate) à Glucose-6-phosphate + ADP (Adenosine Diphosphate)
Enzyme: Hexokinase
Glucose-6-phosphate + NADP+ (Nicotinamide adenine dinucleotide phosphate) à 6-phosphogluconate +
NADPH + H+
Enzyme: glucose-6-phophate dehydrogenase
Methods/ Experimental
Two sport drinks were assigned samples, Powerade and Gatorade. An additional 6 standard drinks – 3
sucrose and 3 glucose- plus 2 blanks were included. Samples were labelled and prepared with dilutions
of sucrose and glucose including a buffer where needed. Using spectrophotometry, wavelength
absorbances and (indirectly) NADPH concentrations were calculated and recorded. TEA and Citrate
buffers were used along with incubators to ensure efficiency of the multi-step enzymatic reaction that
followed. Refer to lab manual for detailed methodology.
Results
Table 1’s absorbance difference column shows the change in NADPH concentrations at the beginning
and end of the experiment. No significant shifts are seen in the data and correlates closely with the
enzymatic reactions occurring after the final 2minutes (A1-A2 columns). In Figure’s 1 and 2 the data was
plot using the standard data only which in turn allowed the calculation of the sample sports drink
figures. This was achieved using the equation for the line of best fit for the standard data – y = 0.168x
and y = 0.1751x respectively. The obtained data was then entered onto the same plot.
S42934048
SCIE3001
3|Page
Table2 shows how the absence of the initial enzyme effects the data as it is not needed to be broken
down. This can be seen by comparing the two A1 columns for Table 1 and 2. The figures in Table 1 are
mostly negative whereas Table 2 depicts positive data. A2 of Table 2 follows a similar trend pattern to
Table 1’s A2 which is indicative to the amount of enzyme activity at the time.
Table1: Sucrose and Glucose Assays
Sample
A1 (340nm)
A2 (340nm)
Absorbance difference (A2 - A1)
Blank
0
0
0
2mM
-0.002
0.421
0.423
4mM
-0.001
0.634
0.634
6mM
-0.004
1.048
1.052
Gatorade
-0.003
0.364
0.367
Powerade
0.012
0.315
0.327
Sample data compared with standards using absorbance
1.2
y = 0.1684x
1
Standards
0.8
0.6
samples
0.4
Linear
(Standards)
0.2
0
0
2Concentration
4 (mM)
6
8
Figure 1: Compared absorbencies in relation to sucrose concentration levels of standard and samples
Table2: Glucose Assays
Sample
Blank
2mM
4mM
6mM
Gatorade
Powerade
A1 (340nm) A2 (340nm)
Absorbance difference (A2 - A1)
0
0
0
-0.002
0.328
0.33
0.012
0.844
0.832
0.003
1.003
1
0.009
0.087
0.078
0.006
0.083
0.77
S42934048
SCIE3001
4|Page
Glucose standards compared with sample data using absorbance
1.2
y = 0.1751x
1
0.8
Standards
0.6
Samples
Linear (Standards)
0.4
0.2
0
0
2
4
Concentration (mM)
6
8
Figure 2: Compared absorbencies in relation to total glucose concentration levels of standard and samples
Table3: Sucrose and Glucose Content of Sports Drinks
Sports
Drink
Gatorade
Sucrose
Glucose
(g/100 mL)
(g/100 mL)
5.48
0.792
Powerade
4.79
0.801
Calculations
S42934048
SCIE3001
5|Page
Discussion
The enzyme reactions were observed based on the breakdown and production of energy. Using
energy it is possible to determine the rate at which enzymes function. The basis for experiment was to
test for the presence of NADPH (energy) at timed intervals. A pre-determined wavelength of 340nm was
used. Recordings for NADPH absorbencies were recorded in Tables 1 and 2 as ‘A1’ and ‘A2’ columns.
Figure 1 and 2 used several standardised figures – 0mM, 2mM, 4mM and 6mM – which allowed a
comparative analysis of the sample data.
By performing the steps outlined in the lab manual, two assays were completed successfully. Table1
presents recordings that were used to compare sucrose and glucose levels whereas Table 2 refers only
to naturally occurring glucose levels. The difference between the two is needed as they both provide
naturally occurring glucose for an immediate energy response in the body – Table 2. Sucrose is made up
of ‘fructose’ (also known as a fruit sugar) and glucose. In order for glucose levels to be obtained the
sucrose was broken down using hydrolysis. (Marieb, 2007). The fructose within the sucrose is needed as
it provides a delayed energy source via the liver’s glycogen cycle due to its lacking adherence initially.
Table 3 shows the sucrose/glucose grams per 100mL for each sample.
The experiment proved to coincide with the nutritional information provided by manufacturers with
minute variation (refer to Table3 and Appendix 1). Repetition would ensure complete accuracy but
deviations are likely to occur otherwise. The enzymes used were assumed to be in an active state after
the 15minute interval and higher concentrations would have ensured efficiency. In addition, the
manufacturer data is an average and varies from bottle to bottle from the factory
S42934048
SCIE3001
6|Page
References
Timberlake, KC 2007, General, Organic, and Biological Chemistry, Pearson Prentice Hall, New Jersey.
Hoehn, K & Marieb, EN 2007, Human Anatomy & Physiology, Pearson Benjamin Cummings, San
Francisco.
Appendix
1. Manufacturer Label:
Powerade is 7.6g/100ml of carbohydrate with 6g of sucrose included.
Gatorade is 6g/100ml of carbohydrate with 5.5g of sucrose included.
S42934048
SCIE3001
7|Page