File
advertisement

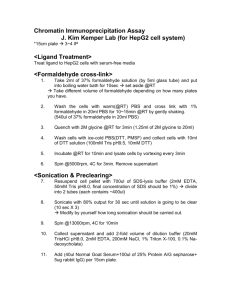
Peqlab RNA/DNA/protein precipitation protocol RNA Isolation 1 For tissue samples: Homogenize the tissue in 1mL TriFast reagent. For cells grown in monolayer: Lyse the cells directly in the culture dish by addition of 1mL TriFast reagent and passing the cell lysate several times through a pipette. (The amount of TriFast reagent required is proportional to the area of the well (1ml/10cm^2); and not on cell density). TIP: Insufficient amount of reagent results in contamination of RNA with DNA. 2 Keep samples at RT for 5 min Add 0.2 ml of pure chloroform per 1ml of TriFast reagent added (without isoamyl alcohol) Shake vigorously (DO NOT vortex) for 15 seconds Keep at RT for 5 minutes. Spin at 12,000g for 5 min at 4*C 3 Take the the aqueous phase (colorless upper phase) for RNA – Save the lower phase for DNA and the interphase for protein at 4*C. Transfer the aqueous phase to a fresh tube. Add 0.5 ml of isopropanol per 1 ml of TriFast added. Keep samples on ice for 10 min Spin at 12,000g for 10 min at 4*C 4 Remove the supernatant gently Wash the RNA precipitate twice with 75% ethanol by vortexing and subsequent centrifugation for 10 min at 12000g at 4*C Air-dry the samples Resuspend the pellet in RNAase-free water Store at -20*C (The A260/280 ration should be 1.6-2.0) DNA Isolation 1 Remove the phenol phase (from step 3) Add 0.3 ml of 100% ethanol per ml of TriFast reagent added Mix well by inversion (DO NOT vortex) Keep at RT for 2-3 min Spin at 2,000g at 4*C for 15 minutes 2 Remove the supernatant (Store at 4*C for protein extraction) To the pellet, add 1 ml of 0.1M Na-citrate in 10% ethanol. Keep at RT for 30 min. Spin at 2,000g at 4*C for 5 min. Repeat this citrate wash step again. 3 Suspend the DNA pellet in 2ml of 75% ethanol. Keep at RT for 15 minutes with periodic mixing (NO vortexing). Spin at 2,000g for 5 min at 4*C 4 Dry the pellet briefly for 10-15 min Dissolve it in 8mM NaOH (about 200 uL) using a wide bore pipette. Adjust the final DNA concentration between 0.2-0.3 ug/ul with 8mM NaOH Protein Isolation 1 To the supernatant from step 2 of DNA isolation: add 1.5 ml isopropanol. Keep at RT for 10 min Spin at 2,000g for 10 min at 4*C 2 Remove supernatant gently Add to the pellet: 2ml solution of 0.3M guanidium hydrochloride in 95% ethanol. Keep at RT for 20 minutes Spin at 7,500g for 5 min at 4*C (Repeat the guanidium hydrochloride washing step thrice) 3 Add 5ml of 100% ethanol to the pellet Keep at RT for 20 min Spin at 7,500g for 5 min at 4*C 4 (Method for large amounts of starting material) Remove ethanol by decantation and air-dry the pellet for 15 minutes Dissolve it in 1% SDS using pipette. Incubation at 50-100*C may be required for complete solubilization. Insoluble material can be removed by centrifuging at 10,000g for 10 min at 4*C. Transfer the supernatant to a fresh tube. This can be used immediately or stired at -20*C for future use. 4’(Method for small amounts of starting material) 1. To extract proteins perform TRIzol® isolation according to the manufacturer's instructions. 2. Protein pellet was dried by centrifuging under vacuum for 10 min as suggested in the TRIzol® protocol 3. Extract the vacuum-dried protein pellet with 2% (w/v) DEA in 50 mM NaCl, at 1:5 or 1:10 ratio (depending on the amount of original material used) for 20–60 min at room temperature. 4. Centrifuge the extracts at 12,000°—g for 10 minutes at 4 °C. 5. Collect the supernatant and neutralize it with 20% volume of 0.5 M Tris–HCl, pH 6.8. 6. Keep at 4 °C for immediate use or at −20 °C for long-term storage. J Biochem Biophys Methods. 2006 Aug 31;68(2):127-31 4” (Method for small amounts of starting material, quite long and expensive) 1) Load the phenol-ethanol supernatants into Spectra/Por® 6 regenerated cellulose (RC) dialysis membranes (MWCO 2000; Spectrum Laboratories, Rancho Dominguez, CA, USA) and dialyze it against three changes of an aqueous 0.1% sodium dodecyl sulfate (SDS) solution at 4°C, changing the solution first after 16 h, then after 4 h, and again after 2 h, respectively. (For every 1 mL phenol-ethanol supernatant, 100 mL 0.1% SDS solution are used). During dialysis, the samples partitioned into three phases: (i) a colorless supernatant (approximately 85% volume), (ii) a globular mass (approximately 10% volume), (iii) a colorless, viscous liquid (approximately 5% volume). 2) Concentrate the supernatant in the dialysis membrane for the purposes of protein content determination by removing samples from the dialysis membrane and loade them into iCON™ Concentrators (20 mL capacity, 9K MWCO; Pierce) and centrifuged at 6000× g at room temperature for 20 min in a swinging-bucket rotor to reduce the volumes from 12 mL to 100 μL. 3) Resuspend the globular mass, containing the bulk of the proteins, in 200 μL total solvent— either 8 M urea in Tris-HCl, pH 8.0, 1% SDS in molecular biology-grade water or a 1:1 combination of the two. BioTechniques 42:467-472 (April 2007)