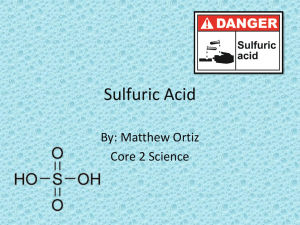
Esters Lab - WilsonSCH4U-02-2012

Michele Zaman
Lab Partner: Elizabeth Williams
Ms. Wilson
SCH4U - 03
Esters Lab
Purpose:
The purpose of this lab was to explore esters. Three esters were prepared and students had to take an educated guess about which odor/scent the esters created were responsible for.
Method:
1.
A hot plate was retrieved and plugged in.
2.
A hot water bath was prepared; the beaker was filled ¼ with water and placed on the hot plate.
3.
The thermometer was placed in the beaker; students were told not to let the hot water bath exceed the temperature of 65 °C.
4.
Students were instructed that one lab partner must stay with the hot water bath at all times
5.
The ester isopentylacetate was prepared under the fume hut. 1.0mL (20 drops) of acetic was mixed with
1.0mL (20 drops) of isopentanol and 3 drops of sulfuric acid was added which is the catalyst. (reaction 1)
6.
The test tube which the ester was mixed in was brought back to the lab bench and placed in the hot water bath
7.
The mixture was to be heated up for minimum of 10 minutes. Students were instructed that the longer the mixture was heated the better results.
*Steps 6-7 were repeated for the 2 nd and 3 rd reaction.
8.
N-octylacetate was prepared under the fume hut. Acetic acid (1.0mL/20 drops) was mixed with n-octanol
(1.0mL/20 drops) and sulfuric acid (3 drops) which is the catalyst was added.
9.
Methylsalicylate was prepared under the fume hut. Salicylic acid (1.0cm high) was mixed with methanol
(1.0mL/20 drops) with and sulfuric acid (3 drops) which is the catalyst was added.
10.
The mixtures in the test tubes were stirred time to time while they were being heated in the hot water bath
11.
The test tubes were retrieved from the hot water bath after they were heated an appropriate amount.
12.
The test tubes were brought a safe distance away from the students face and were wafted so the scent of the ester could be determined.
13.
Saturated sodium carbonate was added to the ester to help students identify the scent.
Materials:
Equipment hot plate
Beaker (250mL) thermometer (1) stirring rod (1) scoopula test tubes(3) test tube rack tape dropper bottles (3) gloves dropper (5) glass containers (5)
1 | P a g e
Chemicals acetic acid salicylic acid methanol n-octanol isopentanol sulfuric acid water sodium carbonate
Michele Zaman
Lab Partner: Elizabeth Williams
Ms. Wilson
SCH4U - 03
Diagram:
2 | P a g e
Michele Zaman
Lab Partner: Elizabeth Williams
Ms. Wilson
SCH4U - 03
Observation:
1.
2.
Ester
Isopentlyacetate n-octylacetate
Quantitative
- acetic acid
(20 drops/
1.0mL)
- isopentanol
(20drops/
1.0mL)
- sulfuric acid
(3 drops)
- acetic acid
20 drops/
1.0mL)
- n-octanol
20drops
/1.0mL)
- sulfuric acid
(3 drops)
3.
methylsalicylate - salicylic acid
(1 cm high)
- methanol
(20drops/1.0mL)
- sulfuric acid
(3 drops)
Before
Sulfuric acid
- red tint, transparent, liquid, strong scent
Acetic acid
- clear, transparent, liquid
Isopentanol
- clear, transparent, liquid
Sulfuric acid
- red tint, transparent, strong scent, liquid
Acetic acid
- clear, transparent, liquid
N-octanol
- clear, transparent, liquid
-Salicylic acid
White, powder like, solid
Sulfuric acid
- red tint, transparent, strong scent, liquid
Methanol
- clear, transparent, liquid
Qualitative
During
-Mixture smelt very strong
- Colourless clear liquid with brownish red liquid at the bottom of the test tube
- Yellowish tint at the top of the test tube
(meniscus)
- Transparent/ clear
-Liquid
-White layer at the bottom of the test tube
-Mixture slowly starts to become cloudy/milky
-Liquid
-Translucent
After
-Transparent
- dull redish/pink tint
- smelt strong and fruity/flowery
Experimental scent: Banana candy
-when sodium carbonate was added, solution bubbled
-Clear, colourless solution
(transparent)
- Strong scent like wax
Experimental scent: Orange peel
-when sodium carbonate was added, solution bubbled
-Mixture starts becoming a clear solution
-Gas bubbles appear on the side of the test tube
-Transparent
-Strong scent
Experimental scent: Minty (life savers)
-when sodium carbonate was added, solution bubbled
3 | P a g e
Michele Zaman
Lab Partner: Elizabeth Williams
Ms. Wilson
SCH4U - 03
Discussion & Analysis:
Follow-Up Questions:
1.
What is the purpose of the hot water bath and concentrated acid?
The catalysts for a condensation reaction with an alcohol and carboxylic acid are sulfuric acid and heat. Therefore the purpose of the concentrated acid (sulfuric acid) and the hot water bath (heat) was to act as the catalyst to speed up the reaction and ensure the products were made.
2.
What is significant about the temperature of 65 °C
During the experiment the students were told not to let the water bath exceed the temperature of 65°C. The significance of this temperature is that it is the boiling point of methanol (CEC, 2012) 1 . Due to the fact that all three test tubes were in the same water bath and the alcohol – methanol was used in the third reaction – the temperature had to stay below 65 degrees. If it were to exceed that temperature methanol would start evaporating, therefore affecting/slowing/stopping the overall reaction because one of the reactants would start disappearing.
3.
Why are all test tubes dry before starting?
The test tubes are all dried before starting the lab because the reaction to formulate these esters is reversible.
When a condensation reaction with an alcohol and carboxylic acid occur the products produced is an ester and water. For the reverse of this reaction which is a hydrolysis reaction the reactants is an ester and water with the catalysts sulfuric acid and heat. Therefore since the reaction is reversible all test tubes must be dry because if there were to be traces of water while the condensation reaction is occurring, after the ester is produced the hydrolysis
(reverse) reaction could start occurring immediately because all the reactants and catalysts would be present. This would tamper with the overall results because the ester would start breaking down.
4.
Use structural or line diagrams to represent each reaction. Include names of all reactants and products and all reaction conditions. Indicate the theoretical odor of each ester produced.
Reaction 1:
O O
OH
O
OH
+ Sulfuric acid
Heat
O
+
H H acetic acid isopentanol isopentylacetate water
Theoretical odor: Banana
4 | P a g e
Michele Zaman
Lab Partner: Elizabeth Williams
Ms. Wilson
SCH4U - 03
Reaction 2:
O
OH acetic acid
+
OH
O sulfuric acid
O
Heat
O
+
H H
n-octanol n-octylacetate water
Theoretical odor: Orange
Reaction 3:
OH
O
OH
O
+ OH Sulfuric acid O
Heat
OH salicylic acid methanol methylsalicylate
Theoretical odor: Oil of winter green (chemicalland21, 2012) 2
+
H
O
water
H
Esterification Reactions:
1.
The ester responsible for the odour of a lily is 2-phenylethlypropanate
OH
O
OH
+
Sulfuric acid
O
+
O
Heat
O H H propanoic phenylethanol 2-phenylethylpropanoate water
H O
2.
The odour of pineapple is characteristic of an ester produced from n-butanol and butanoic acid
O
O
Sulfuric acid
O
+
H O
Heat
+
H H n-butanol butanoic acid butylbutanoate water
5 | P a g e
Michele Zaman
Lab Partner: Elizabeth Williams
Ms. Wilson
SCH4U - 03
3.
Ethylcinnamate is the ester responsible for the scent of cinnamon. The IUPAC name for cinnamic acid is 3-phenylprop-2-enoic acid.
O
OH +
OH
Sulfuric acid
Heat
O
O +
H
O
H
cinnamic acid ethanol ethylcinnamate water
(3-phenylprop-2-enoic acid)
Conclusion:
In conclusion this lab was over all very successful. During this lab was students had to work with great precaution because this was the first lab concentrated acids were handled directly. In the end, the safety rules were followed and the lab was done smoothly, allowing the experimenters to explore the world of esters. All of the esters required were made successfully and with a slight bit of struggle including lots of wafting the scents of the esters were identified correctly.
Reaction 1: theoretical – banana, experimental – banana candy
Reaction 2: theoretical – orange, experimental – orange peel
Reaction 3: theoretical – oil of winter green, experimental – minty (life savers)
What was learned through preparing these esters was the comprehension of how an ester reaction works. There is a big difference between reading about how a reaction works and physically mixing in the reactants and receiving a product. Through exploring esters and receiving this knowledge, it made answering all of the discussion questions possible.
Source of errors: There are many possible sources of error in this lab such as: the test tubes or beakers being contaminated prior to the experiment, or the beaker and test tubes having residue left from previous lab that washing did not get rid of. The test tubes may still have had slight traces of water in them. The thermometer used for the hot water bath may have been faulty by displaying in accurate readings of the temperature. Also there may have been something wrong with the chemicals used because of where they were manufactured.
6 | P a g e
Michele Zaman
Lab Partner: Elizabeth Williams
Ms. Wilson
SCH4U - 03
References:
2 "METHYL SALICYLATE (BETULA OIL)." Chemicalland21.com =Chemical Answer= . N.p., n.d. Web. 20
Nov. 2012. <http://chemicalland21.com/lifescience/foco/METHYL%20SALICYLATE.htm
1 "PHYSICAL PROPERTIES OF METHANOL- Cetiner Engineering." CETINER ENGINEERING
CORPORATION, methanol, ammonia . N.p., n.d. Web. 20 Nov. 2012.
<http://www.cetinerengineering.com//Properties.htm
1.
Personal communication with Elizabeth Williams. November 20 th 2012
2.
Personal communication with Ms. Wilson. November 20 th 2012
7 | P a g e
Michele Zaman
Lab Partner: Elizabeth Williams
Ms. Wilson
SCH4U - 03
8 | P a g e