Unit 6b Notes: Oxidation Numbers, Reactions in Aqueous Solutions
Unit 6b Notes: Oxidation Numbers, Reactions in Aqueous
Solutions, & Predicting Products
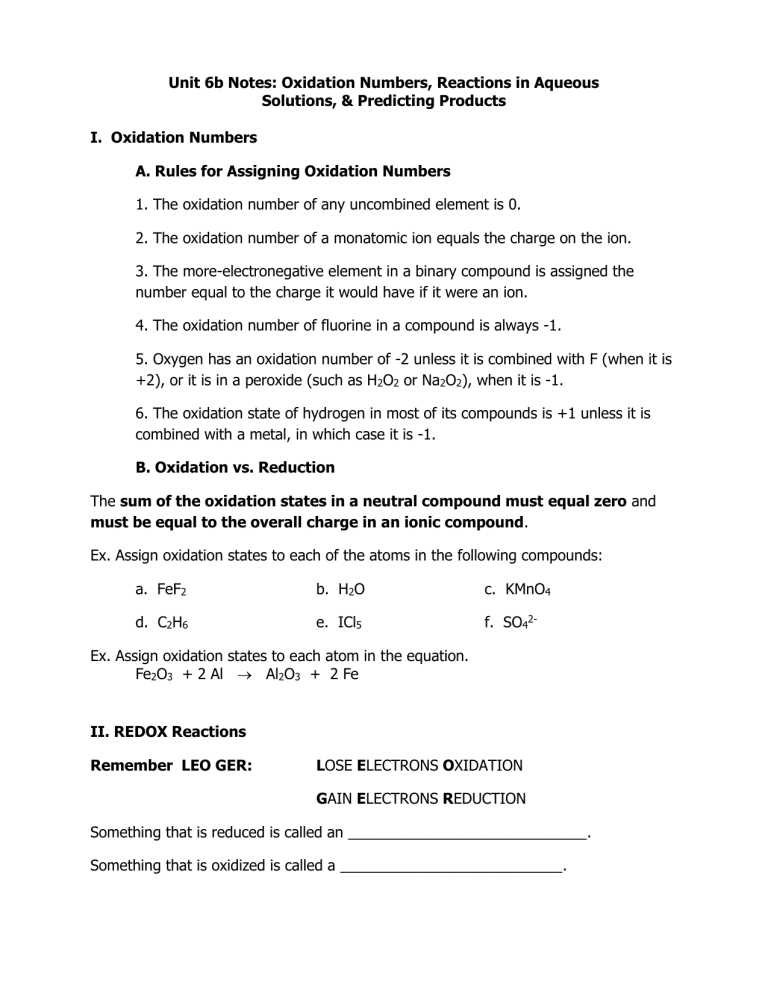
I. Oxidation Numbers
A. Rules for Assigning Oxidation Numbers
1. The oxidation number of any uncombined element is 0.
2. The oxidation number of a monatomic ion equals the charge on the ion.
3. The more-electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion.
4. The oxidation number of fluorine in a compound is always -1.
5. Oxygen has an oxidation number of -2 unless it is combined with F (when it is
+2), or it is in a peroxide (such as H
2
O
2
or Na
2
O
2
), when it is -1.
6. The oxidation state of hydrogen in most of its compounds is +1 unless it is combined with a metal, in which case it is -1.
B. Oxidation vs. Reduction
The sum of the oxidation states in a neutral compound must equal zero and
must be equal to the overall charge in an ionic compound.
Ex. Assign oxidation states to each of the atoms in the following compounds: a. FeF
2 b. H
2
O c. KMnO
4 d. C
2
H
6 e. ICl
5
f. SO
4
2-
Ex. Assign oxidation states to each atom in the equation.
Fe
2
O
3
+ 2 Al Al
2
O
3
+ 2 Fe
II. REDOX Reactions
Remember LEO GER: LOSE ELECTRONS OXIDATION
GAIN ELECTRONS REDUCTION
Something that is reduced is called an _____________________________.
Something that is oxidized is called a ___________________________.
Ex. For each reaction, identify the atoms that undergo reduction and oxidation. a.
2 H
2 (g)
+ O
2 (g)
2 H
2
O
(g) b.
Zn
(s)
+ Cu 2+
(aq)
Zn 2+
(aq)
+ Cu
(s) c.
2 AgCl
(s)
+ H
2 (g)
2 H +
(aq)
+ 2 Ag
(s)
+ 2 Cl -
(aq) d. 2 MnO
4
-
(aq)
+ 16 H +
(aq)
+ 5 C
2
O
4
2-
(aq)
2 Mn 2+
(aq)
+ 10 CO
2(g)
+ 8 H
2
O
(l)
Learning Check – Oxidation Numbers
Assign oxidation states to each of the atoms in the following compounds: a. SO
2 b. S c. SO
3
Assign oxidation states to each atom in the equation. Determine which element was oxidized and which was reduced.
2 Al + 3 CuCl
2
2 AlCl
3
+ 3 Cu
III. Dissociation
When a soluble ionic salt dissolves in water the ions separate and a hydration shell is formed around each ion (Dissociation)
EX. Ba(NO
3
)
2
in water –
Aluminum acetate
Aluminum carbonate
EX. Draw a beaker of dissociated sodium chloride; a beaker of dissociated Aluminum nitrate; and a beaker of silver chloride.
Learning check- Dissociation
Write a dissociation equation for aluminum sulfate
Draw a beaker of dissociated aluminum sulfate
IV. Predicting Products
A. Single Displacement reactions: an element and a compound combine to form a new element and compound.
* Use the activity series.
General Equation:
Example:
Active metal elements can replace less active metals; active nonmetal elements can replace less active nonmetals. Use the Activity series (snoopy sheet) to determine whether or not the reaction will occur. Driving force is the transfer of electrons.
Learning check – Single Displacement copper + silver nitrate bromine + sodium chloride
B. Double Displacement reactions: two compounds combine to produce two different compounds - Acid-Base and Precipitation Reactions.
* Use solubility rules.
General Equation:
Example:
Learning check – Double Displacement sodium sulfate + lead (II) nitrate sulfuric acid + potassium hydroxide
Describing Aqueous Reactions–Molecular, Complete and Net Ionic Equations
Molecular Equation: shows the complete formula of all reactants and products
Ex: Silver nitrate + barium chloride
Complete Ionic Equation: represents aqueous compounds as ions
Ex:
Net Ionic Equation: includes only those components directly involved in the reaction. Ions present on both sides on the equation and do not participate directly in the reaction are called Spectator Ions
Ex:
Ex: Write the molecular, complete ionic and net ionic equations for the following reaction.
Sodium + Zinc Nitrate
C.
Decomposition reactions: a single compound is broken down into more than one product. There are six different types.
1. Decomposition of a binary compound into its elements.
* Usually requires heat or electricity.
General Equation:
Example:
2. Decomposition of a base into a metal-oxide and water.
General Equation:
Example:
3. Decomposition of a ternary acid into a nonmetal-oxide and water.
General Equation:
Example:
4. Decomposition of a metallic carbonate into a metal-oxide and carbon dioxide.
General Equation:
Example:
5. Decomposition of a metallic chlorate into a metal-chloride and oxygen gas.
General Equation:
Example:
6. Decomposition of a ternary salt into a metal-oxide and a non-metal oxide
General Equation:
Example:
Special Situations
Whenever H
2
CO
3
, H
2
SO
3
, or NH
4
OH is a product it will decompose immediately as follows: H
2
CO
3
H
2
O + CO
2
H
2
SO
3
H
2
O + SO
2
NH
4
OH H
2
O + NH
3
Learning check - Decomposition
Calcium chlorate
Phosphoric acid
Barium hydroxide
Tin (IV) carbonate
D.
Synthesis reactions: two substances combine to form one product. There are four different types.
1. Two elements combine to form a binary compound.
General Equation:
Example:
2. Combining a metal-oxide and water to produce a base.
General Equation:
Example:
3. Combining a nonmetal–oxide and water to produce a ternary acid.
General Equation:
Example:
4. Combining a metal-oxide and a nonmetal-oxide to produce a ternary salt.
General Equation:
Example:
Learning check- Synthesis
Water + magnesium oxide
Water + dinitrogen trioxide
Bromine + sodium
E.
Combustion reactions: Certain organic compounds (Hydrocarbons – compound containing Carbon and Hydrogen or Carbon, Hydrogen and Oxygen) burn to produce specific products. There are two types.
1. Complete combustion – combining a hydrocarbon with excess oxygen to produce carbon dioxide and water.* if the equation does not indicate limited oxygen assume complete combustion
General Equation:
Example:
2. Incomplete combustion - combining a hydrocarbon with limited oxygen to produce carbon monoxide and water.
General Equation:
Example:
Learning check - Combustion
C
8
H
18
+ oxygen
C
2
H
2
+ oxygen
V. Reaction Rates
Reaction rate depends on the collisions between reacting particles.
A.
Successful collisions occur if the particles...
_______________ with each other
have the correct _________________________________
have enough _____________________ to break bonds
B.
To speed up the rate of the reaction:
Increase _____________________ (smaller particles or dissolve in water)
Increase _____________________ (add more reactant)
Increase _____________________ (add heat source)
Add __________________________________________
VI. Heat in Reactions
A.
Exothermic reactions release heat
Heat is a product
Feels hot
B.
Endothermic reactions absorb heat
Heat is a reactant
Feels cold