1 - University of Waterloo
advertisement

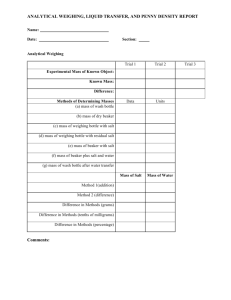
Getting More Activity Out Of the Activity Series Additional Information Presented at ChemEd 2013 Chris Miedema Ashbury College Ottawa, Ontario CANADA cmiedema@ashbury.ca Thanks to Debbie Goodwin (Master Teacher), Dr. Winston Revie (National Resources Canada-Retired) plus the ASM Foundation and NACE Foundation for all their assistance in my professional development. Cost of a Penny I include this as I assume we have participants from both sides of the border. Unfortunately, for Canadians this is a difficult lab to do for two reasons. (1) Canada has eliminated the penny from its currency, and (2) the Canadian penny had a different core from American ones . Check the Canadian Mint site for the composition history. (http://www.mint.ca/store/mint/learn/1-cent5300004#.UfQMghb3WaA) Day #1 1. Make a table to record your data. Record the date of a pre-1982 penny and a post-1982 penny. 2. Measure and record the thickness (using the digital calipers) and the mass (centigram balance) for each penny. Be sure to use labels. 3. Use sandpaper to remove a thin layer of copper around the rim of the penny make sure that you see silver-colored zinc exposed on the post-82 pennies. Or use a triangular file to make four 1mm deep scores in the penny’s edge at 90 degrees apart. 4. Place the pennies in a beaker or plastic (PS) cup and add 20 - 25 mL of 6M HCl. 5. Place in the fume hood or by an open window. 6. Write observations. Day #2 1. Decant the HCl into the sink, flushing with plenty of water. Add about 50 mL of tap water to the beaker to rinse off the pennies. Pour the water off and tip the pennies onto a paper towel to dry. 2. Measure and record the thickness and mass of each penny. Write observations about changes in appearance of each penny. 3. Answer the “Questions and Calculations” on the handout. 4. Write a final summary paragraph and a reflection paragraph. Safety Safety glasses/goggles must be worn during this experiment. The beaker containing the 6M HCL should be placed in a fume hood or by an open window overnight. From Everything is Material ASM International Foundation p 14-M Aluminum Can Demo Remove the paint from an unopened pop can o Use a scotchbrite pad (found in the paint department at Wal-Mart or Lowe’s) o Using an unopened pop can makes it much easier to remove the paint Empty the pop out of the can and rinse Poke 2 holes on opposite sides of the can near the top o Run a copper wire through the holes to suspend the can in a beaker o OR use a stirring rod to suspend the can by the tab Fill the can with cold water (the reaction to remove the aluminum is exothermic) Suspend the can in a 600 mL beaker Gradually add 3M HCl or 1M NaOH to the beaker o Add between a half inch and an inch at a time o Once the reaction stops, add another ½” to 1” of solution o Concentrated copper II chloride solution or copper II sulfate (with NaCl added) solution may be used o HCl is “cleaner” than NaOH Gently rinse the can in a beaker of water Notes: Use safety precautions when using chemicals. Using HCl will produce hydrogen gas as a by-product and is an exothermic reaction. Wear appropriate lab safety gear such as safety glasses and lab aprons and consult specific MSDS. Adding too much HCl or NaOH at a time may cause the polymer liner to melt from the heat generated or rip due to vigorous bubbling Masking tape can be used to make a design on the can, such as stripes or letters, before sanding. The polymer liner is spray-coated onto the inside of the can during production. It is to protect the aluminum from the phosphoric acid that pop contains. The chemical reaction that removes the aluminum by using HCl is a simple single-replacement reaction: 6HCl + 2Al ----- 3H2 + 2AlCl3 This demonstration comes from the materials provided to participants of the ASM Materials Camp for teachers. See http://www.asminternational.org/portal/site/www/foundation/educators/teache r-material-camp/ for more details. Corrosion Materials City of Ottawa Sacrificial Anode Program: See: http://ottawa.ca/en/residents/water-and-environment/drinking-water-andwells/extending-life-watermains-your-community The following reports giving background information from other areas are also available on the internet: AWWA, “Dawn of the Replacement Era: Reinvesting in Drinking Water Infrastructure,” AWWA, Denver, CO, 2001. www.win-water.org/reports/infrastructure.pdf S. Folkman, “Water Main Break Rates in the USA and Canada: A Comprehensive Study,” Utah State University Buried Structures Laboratory, April, 2012 http://www.watermainbreakclock.com/docs/UtahStateWaterBreakRates_FINAL_T H_Ver5lowrez.pdf C. Holloway, “Cathodic Protection Program (1983 – 2007) 2007 Annual Report” Region of Durham Works Department, 2007 http://www.corrosionservices.us.com/Durham_Ontario_2007_Annual_Report.pdf Thanks also to Mr. Wayne Shields and Ms. Sandy Yenson for their helpful discussions. Pitting Corrosion. See the book Ink Sandwiches, Electric Worms and 37 Other Experiments for Saturday Science by Neil A. Downie. P 308 entitled “Unusually Cool Sunglasses” for details. (I love Downie’s books by the way) Stray Current Corrosion We got started with the following: http://www.corrosionservices.us.com/Durham_Ontario_2007_Annual_Report.pdf http://www.ce.sc.edu/deptinfo/members/faculty/ray/web1/ugrad/eciv%20303/ Corrosion/Set7%20CorrosionCells.pdf We also looked at “The Other Stray Current Corrosion” by Dick Troberg found in Professional Boatbuilder No 105, February/March 2007. This article suggests a number of experiments as well. Your library data base might have this journal on it. Raku Pottery This project has been one of the best I have encountered for student interest and excitement. Plus they learn something from it. If the students take the project home, they usually talk about it favorably with their parents, and you know the benefits of that! I have attached the method to this document. The source of this material is from documents provided during the ASM Teacher Camp for Teachers. See http://www.asminternational.org/portal/site/www/foundation/educators/teache r-material-camp/ for more details. Students taking Chemistry that also take Art occasionally ask to do more comprehensive work in this area. Our Art teacher has taken to purchasing the clay. You do need a front -loading kiln. For schools that cannot afford one, I always wondered whether you could strike a deal with a local pottery club. Use of their kiln for the possibility of attracting new, young, members. Cleaning Silver This method can be found all over the internet. Here are the basics: 1. Heat 400 ml of water 2. Place sheet of Al foil so it covers the bottom of a second beaker. Alternatively, you could just use an Al pan or dish. (The latter is what I do) 3. Place the tarnished silver in the container so it is touching the foil. 4. Mix (1:1) salt/baking soda and stir into the boiled water until dissolved. 5. Pour the hot solution over the tarnished silver 6. After a few minutes, remove the silver and record any observations. 7. This could be an open ended activity by studying different conditions and salt. I usually break my class up into small groups and have them do several activities and then explain what they have done rather than getting this formally written up. Participants of the ASM Materials camps get a copy of this lab and several others in a booklet form as part of a free cKit they receive.