Kristin Miller
advertisement

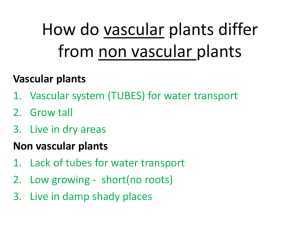
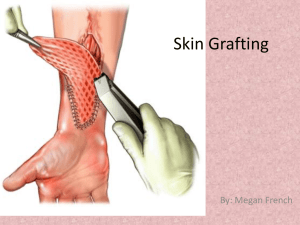
Evaluating the Growth Potential of Tissue Engineered Vascular Grafts: Role of Vasoactivity and Active Endothelium to Altered Mechanical Stimuli Kristin S. Miller Ph.D.1, Ramak Khosravi B.S.2, Christopher K. Breuer M.D.3, Jay D. Humphrey Ph.D.2,4 1Tulane University, New Orleans, LA, 2Yale University, New Haven, CT, 3Nationwide Children’s Hospital, Columbus, OH, 4Yale School of Medicine, New Haven, CT Continued advances in the tissue engineering of vascular grafts have enabled a paradigm shift from the desire to design for adequate suture retention, burst pressure, and thrombo-resistance to the goal of minimizing compliance mismatch between the grafts and adjacent vasculature. Toward this end, we recently presented a modeling framework that predicts salient features of neovessel evolution considering the monotonic loss of a load-bearing polymer scaffold and the subsequent production of collagen and passive smooth muscle. We have shown that the computational framework is amenable to evaluate compliance mismatch as well as to assess the consequences of different scaffold parameters on neovessel formation. The growth potential of vascular grafts to adapt to altered hemodynamics, however, has not yet been evaluated. Vasoactivity and an active endothelium are thought to be integral to vascular adaptation. Despite continued advances in vascular tissue engineering, however, experiments have not yet yielded neovessels with active smooth muscle. Toward this end, in this study we evaluate in silico the growth potential of interposition grafts, implanted within the murine venous circulation, to altered hemodynamics and axial stretch following the formation of a neovessel (steady remodeled state). Further, the potential contributions of active smooth muscle and an active endothelium to neovessel adaptation are evaluated. We submit that growth and remodeling models enable hypothesis-driven studies to evaluate different methods of graft failure (e.g., lack of growth potential and compliance mismatch). Computational models should thus be viewed as valuable tools to screen scaffold designs and guide experiments, and thus advance vascular tissue engineering.