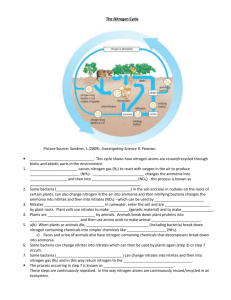
Where is nitrogen found in the environment? The largest single
advertisement

Where is nitrogen found in the environment? The largest single source of nitrogen is ________________________ Nitrogen makes up _____ of our air! What happens to atmospheric nitrogen (N2) in the nitrogen cycle? Atmospheric nitrogen is converted to _______________________________. Why does atmospheric nitrogen need to be converted? It is one of nature’s great ironies… Nitrogen is an essential component of _____, _____ and _______—the building blocks of life. Although the majority of the air we breathe is nitrogen, most living organisms are unable to use nitrogen as it exists in the atmosphere! How does atmospheric nitrogen get changed into a form that can be used by most living organisms? By traveling through one of the four processes in the Nitrogen Cycle! (1) _________________________________ (2) _________________________________ (3) _________________________________ (4) _________________________________ The first process in the nitrogen cycle is… ________________________! What is “nitrogen fixation” and what does it mean to say nitrogen gets “fixed”? “Nitrogen Fixation” is the process that breaks apart the N2 molecules found in the atmosphere so they can combine with oxygen or hydrogen. There are three ways that nitrogen gets “fixed”! (a) ______________________ (b) ______________________ (c) ________________________ Atmospheric Fixation (Only 5 to 8% of the Fixation Process) The enormous energy of _________________________________________________ and enables the nitrogen atoms to combine with oxygen forming nitrogen oxides (N2O). Nitrogen oxides dissolve in rain, forming nitrates. Nitrates (NO3) are carried to the ground with the rain for ____________________________________. Industrial Fixation Under great pressure, at a temperature of 600 degrees Celsius, and with the use of a catalyst, atmospheric nitrogen (N2) and hydrogen are combined to form ammonia (NH3). Ammonia can be used as a _______________. Biological Fixation (__________________________________________) There are two types of “Nitrogen Fixing Bacteria” Free Living Bacteria (“fixes” 30% of N2) Symbiotic Relationship Bacteria (“fixes” 70% of N2) Free Living Bacteria Highly specialized bacteria live in the soil and have the ability to combine atmospheric nitrogen with hydrogen to make ammonia (NH3). Symbiotic Relationship Bacteria Bacteria live in the roots of legume family plants and provide the plants with ammonia (NH3) in exchange for the plant’s carbon and a protected home. Most atmospheric nitrogen (N2) is “fixed” and changed to ammonia (NH3). Ammonia is highly toxic to many organisms. Can plants use ammonia? Very few plants can use ammonia (NH3)… …but, fortunately the second process Ammonification can help! What is ammonification? Ammonification: ___________________________________________________________ _________________________________________________________________________ What happens to ammonium (NH4) stored in the soil? It travels through the third process of the nitrogen cycle called Nitrification! Nitrifying bacteria in the ground convert the ammonium to nitrites then nitrates which green plants can absorb and use! How does nitrogen re-enter the atmosphere in the nitrogen cycle? Through the fourth process called denitrification! What does denitrification do? Denitrification converts nitrates (NO3) in the soil to atmospheric nitrogen (N2) replenishing the atmosphere How does the denitrification process work? Denitrifying bacteria live deep in soil and in aquatic sediments where conditions make it difficult for them to get oxygen. The denitrifying bacteria use _____________________ ______________________________________________________________________, leaving free nitrogen gas as a byproduct. They close the nitrogen cycle! Other ways that nitrogen returns to the atmosphere… ______________________________________ and ___________________________ create nitrous oxides gas (N2O). ____________________________ emit nitrous oxides gas (N2O).