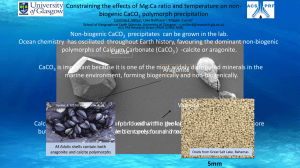
PHREEQC Tutorial: Water Chemistry & Speciation Modeling
advertisement

PHREEQC Getting Started: Download program at the following webpage: HTTP://wwwbrr.cr.usgs.gov/projects/GWC_coupled/phreeqc Read through information on web page. There is much useful information including multiple version (PC, Mac, Linux) and help information Download the most current version. As of this March 2015, the version is o PhreeqcI version 3.1.7-9213 Note: Phreeqc is updated often. Be sure to use the most recent version. There also may be an issue with a conflict with windows. If so, there is a simple explanation for how to download a patch on the download page. A few examples of things that PHREEQC can do. These notes provide a few examples of what the program can do in terms of calculations of water chemistry, water-rock interactions, and speciation of water. The program has many more functions, but it is intuitive and easy to use and once you are able to input data and extract results, you will be able to run many types of simulations. There is also a very good manual with multiple examples that you can work through to develop other simulations to try. I. Speciation Modeling: Calculate saturation states, complexation, and species present given some water composition: Given water with the following composition: 1 Solution 1. Ion Ca Mg Na K HCO3 SO4 Conc. (mg/L) 48 3.6 2.1 1.2 152 3.2 Ion Cl NO3 Fe pH T Conc. (mg/L) 8.0 0.3 0.05 7.5 20 Questions to be addressed about this water: (1) What complexes and species are present in the water? (2) What are saturation states of various minerals? A. Procedure: (1) Click on “Solution” button (a picture of a small flask) (2) Fill in data where needed. For this example, you will first provide T and pH under the first tab on the sheet. Then move to “individual element input” tab. Include all the data. Note: a) be sure to include correct units. You may use “Default Units” or provide units individually. All of Phreeqc calculations and reports are molar concentrations. The program will automatically convert to molar units for you. b) You may calculate the charge balance, or may correct for charge balance with some ion, for example bicarbonate if you are missing that analysis. c) Not that the program automatically includes the mass of water as 1 kg. That can be changed, but the default is to react all solutions as 1 kg of water. (3) Place cursor in the window on the right window. This window should now show the values of the data that you have input. This will highlight the blue “Run…” button. Click button. You may be prompted to save, or if you have previously saved the file, it will warn you that you will overwrite the previous file. (4) Click Start. The calculations will be made. Click dismiss. At this point the tabs at the bottom of the left page will change from blue “input” tab to green “output” tab. And data will be shown. B. Results: When you shift to the output tab, you will be able to open folders by clicking on the “+” symbol next to any folder or symbol. Opening the folder next to the 2 “Beginning of initial solution calculation” gives another folder that is the “Initial solution 1”. This will open up and allow access to the four sections of the data results including: Solution composition Description of Solution Distribution of Species Saturation indices By clicking on any one of these buttons will take you to that point of the data results. Solution composition This button shows the composition of the input solution, but concentrations have now been converted to molality and it shows the number of moles of each input solute. The values for molality and number of moles are identical in this simulation since the solutes are dissolved in 1 kg of water. Description of Solution This button shows the characteristics of the solution, including those values that were input initially, including pH, pe, and temperature. Much of the data shown here are derived values including: specific conductance, density, volume, activity of water, ionic strength, mass of water, total carbon and total CO2 (in this example, identical since the only C came from alkalinity), electrical charge balance, an the present error. Description of Species For each element, the total concentration of that element is listed, and then below each element information about that element is included in the list. This information includes: molality, activity, log values of these two variables as well as the activity coefficient and the molar volume of the species. For example: Species Ca Ca+2 CaHCO3+ CaCO3 CaSO4 CaOH+ CaHSO4+ Molality 1.198e-003 1.155e-003 3.322e-005 6.046e-006 3.813e-006 4.929e-009 7.592e-013 Activity 8.748e-004 3.099e-005 6.052e-006 3.817e-006 4.590e-009 7.071e-013 Log Molality Log Activity -2.937 -4.479 -5.219 -5.419 -8.307 -12.120 -3.058 -4.509 -5.218 -5.418 -8.338 -12.150 Log Gamma -0.121 -0.030 0.000 0.000 -0.031 -0.031 For example, the Ca concentrations is about 1.2 x10-3 molar and there are several dissolved species present including CaHCO3 (3.322 x 10-5 molar), CaCO3 (6.046 x 10-6 molar), CaSO4 (3.813x 10-6 molar) etc. 3 Saturation indices The last value includes the saturation indices relative to all of the mineral phases for which thermodynamic data are available in the data base you are using (more later) and for which elements are provided in the solute composition list. Four our example: ------------------------------Saturation indices------------------------------Phase Anhydrite Aragonite Calcite CO2(g) Dolomite Gypsum H2(g) H2O(g) Halite Melanterite O2(g) Siderite SI log IAP -3.36 -0.11 0.04 -2.24 -0.70 -3.14 -23.00 -1.51 -9.33 -8.74 -37.19 -0.78 -7.72 -8.44 -8.44 -3.70 -17.79 -7.72 -26.15 -0.00 -7.75 -10.95 -40.08 -11.67 log KT -4.36 -8.34 -8.48 -1.47 -17.09 -4.58 -3.15 1.51 1.58 -2.21 -2.89 -10.89 CaSO4 CaCO3 CaCO3 CO2 CaMg(CO3)2 CaSO4:2H2O H2 H2O NaCl FeSO4:7H2O O2 FeCO3 This file shows each possible mineral phase, it’s Saturation Index, the log of the ion activity product (IAP), and the log of the equilibrium constant for the temperature of the water (KT). The last column is the stoichiometry of the mineral phase that is equilibrium. For example: on this page, aragonite is slightly undersaturated (SI = -0.11), but Calcite is slightly oversaturated (SI = 0.04). II. Changing Data bases When you ran the program in part I, one of the menu items that popped up was called Run. That menu gave you the option of indicating which Input file, Output file and the Database file to run. The database includes all the thermodynamic data used to make the saturation calculations and parameters for estimating the activity coefficients. To change the database used in the calculations, double click on the radio button with the three dots located to the right of the scroll down menu. This button will open the Phreeqc Interactive folder to the group of .dat files. The default file is set to phreeqc.dat, but you should have a choice of several others. You will also have the option of resetting the default file to any .dat file you choose. The different files have been compiled for other speciation software, other laboratories, or gathered from the literature. You may look at the structure of the .dat files and change them directly in the files. Go to My Computer, in the C drive and look for Phreeqc Interactive folder. 4 Note: this folder may be located in a folder labeled USGS. Open the Phreeqc Interactive folder and there should be several .dat files along with the phreeqci.exe file, a folder with examples, and doc folder with the help manual, among others. Right click one of the .dat files and open it with the WordPad program. In this file you will find all the solutions species, logK values, coefficients for calculating activity coefficients using the Debye-Huckel formula. Although you can change, add, or delete parameters in this file, it is safer to do this through the program when adding data to simulate. Do the following procedure to observe how much the data base changes. (1) With the data that was input as simulation one, run the simulation again, but this time change the .dat file to the llnl.dat (data from Lawrence Livermore National Laboratory). (2) Scroll down to the Saturation indices to observe calculations of saturation states of the various minerals. You will see several things have changed, including an increase in number of minerals for which saturation states have been estimated. Saturation indices can be calculated for more minerals because of the larger amount of elemental data in the llnl.dat files; saturation indices cannot be calculated without the constituents available. Saturation states of the minerals have also changed, for example, SI aragonite is now -0.16, down from -0.11. For dolomite, there are now calculations of different types including stoichiometric dolomite, ordered dolomite and disordered dolomite. 5 III. Batch Reaction Modeling: What happens if two waters with different compositions mix? Given another water with the following composition: Table 2. Solution 2 Ion Ca Mg Na K HCO3 SO4 Conc. (mg/L) 96 7.2 4.2 2.4 304 6.4 Ion Cl NO3 Fe pH T Conc. (mg/L) 16.0 0.6 0.1 8.0 25 A. Some questions: (1) What is the composition of water derived from mixing specified amounts of two different kinds of water? (2) What are some of the species present in the mixed water? (3) How do the saturation states of various minerals change? B. Procedure: (1) Go back to the “input” tab on the lower left window. (2) Highlight “simulation” from the first simulation. Place an “end” at the end of the simulation by clicking on the black dot in the menu. (3) Make sure that “end” is highlighted and click on the solution button once more to include a second solution. Input the data into the spreadsheet for this solution similar to the first solution. (4) Highlight “end” again and click on the mix button – a red volumetric flask with a plus (+) connected to it. A menu will open and should show radio buttons to highlight both solutions. (5) Click on the first and second solution. It will give a value of 1 for the mixing proportions for each water. This mixing solution essentially represents 1 L for each solution (e.g. 50% of each). You can change the proportions to any proportion you wish – it does not have to add to 100%. For this example, mix the two solutions in proportion of 4:1, e.g., “4” of solution 1 and “1” of solution 2. (e.g., 80% solution 1, 20% solution 2). 6 (6) Place cursor in the window on the right. This will highlight the blue “Run…” button. Click button. You may be prompted to save, or if you have previously saved the file, it will warn you that you will overwrite the previous file. (7) Click Run. The calculations will be made. At this point the tabs at the bottom of the left page will change from blue “input” tab to green “output” tab and data will be shown. C. Results: Open the folder called “Reading Input Data from Solution 1”. If you open all the files it will allow you to look through all the calculations, which will show the speciations of the two solutions, and saturation states of all possible minerals. Open the “Batch Reactions” button, which is a large dark dot with Rx in white. (1) In this file, it will show the composition of the mixing proportions of the mixed water as well as the composition of the mixed water determined from the Batch reaction. Note, your results may differ from these presented below, for example, the Specific conductance and volume of water not shown, since that is a new feature in the model. These were calculated using an older version of the model Reaction step 1. Using mix 1. Mixture 1. 4.000e+000 Solution 1 1.000e+000 Solution 2 -----------------------------Solution composition-----------------------------Elements C Ca Cl Fe K Mg N Na S Molality Moles 5.237e-003 1.678e-003 4.798e-004 1.577e-006 3.070e-005 5.714e-004 8.860e-005 3.256e-004 1.517e-004 2.618e-002 8.388e-003 2.399e-003 7.884e-006 1.535e-004 2.857e-003 4.430e-004 1.628e-003 7.585e-004 ----------------------------Description of solution---------------------------pH pe = = 7.458 11.620 Activity of water = 1.000 Charge balance Adjusted to redox equilibrium 7 Ionic strength Mass of water (kg) Total alkalinity (eq/kg) Total CO2 (mol/kg) Temperature (deg C) Electrical balance (eq) Percent error, 100*(Cat-|An|)/(Cat+|An|) Iterations Total H Total O = 7.402e-003 = 5.000e+000 = 4.917e-003 = 5.237e-003 = 25.000 = -4.665e-003 = -9.06 = 20 = 5.550865e+002 = 2.776123e+002 Note that in the description of the water it shows the percentage error based on the charge imbalance. (2) It will have the distribution of species; shown here are just the H2O, C, and Ca species. Again, the list has the concentrations listed in terms of their molality, activities, log molality, log activity and the activity coefficients: ----------------------------Distribution of species---------------------------Species Molality Activity Log Molality Log Activity Log Gamma OHH+ H2O C(-4) CH4 C(4) HCO3CaHCO3+ CO2 MgHCO3+ CaCO3 CO3-2 MgCO3 NaHCO3 NaCO3FeHCO3+ FeCO3 Ca Ca+2 CaHCO3+ CaSO4 CaCO3 CaOH+ CaHSO4+ 4.553e-007 3.637e-008 5.551e+001 0.000e+000 0.000e+000 1.303e-001 1.108e-001 8.588e-003 5.537e-003 3.122e-003 1.341e-003 3.819e-004 2.998e-004 1.997e-004 1.409e-005 1.092e-012 3.083e-013 2.976e-002 1.682e-002 8.588e-003 3.013e-003 1.341e-003 4.431e-008 7.031e-010 3.370e-007 2.961e-008 9.969e-001 -6.342 -7.439 1.744 -6.472 -7.529 -0.001 -0.131 -0.089 0.000 0.000e+000 -130.715 -130.702 0.013 8.547e-002 6.627e-003 5.708e-003 2.393e-003 1.382e-003 1.354e-004 3.090e-004 2.059e-004 1.080e-005 8.365e-013 3.178e-013 -0.956 -2.066 -2.257 -2.506 -2.873 -3.418 -3.523 -3.700 -4.851 -11.962 -12.511 -1.068 -2.179 -2.244 -2.621 -2.860 -3.868 -3.510 -3.686 -4.967 -12.078 -12.498 -0.113 -0.113 0.013 -0.116 0.013 -0.450 0.013 0.013 -0.116 -0.116 0.013 6.077e-003 6.627e-003 3.106e-003 1.382e-003 3.395e-008 5.387e-010 -1.774 -2.066 -2.521 -2.873 -7.354 -9.153 -2.216 -2.179 -2.508 -2.860 -7.469 -9.269 -0.442 -0.113 0.013 0.013 -0.116 -0.116 (3) Finally at the bottom of the page, under the tab for “Saturation indices” it will show the saturation state of the mixed water. Phase SI log IAP log KT 8 Anhydrite Aragonite Calcite CH4(g) CO2(g) Dolomite Fe(OH)3(a) FeS(ppt) Goethite Gypsum H2(g) H2O(g) H2S(g) Halite Hematite Jarosite-K Mackinawite Melanterite N2(g) NH3(g) O2(g) Pyrite Siderite Sulfur -0.45 2.25 2.40 -127.84 -0.78 4.52 3.24 -130.88 9.13 -0.23 -38.21 -1.51 -128.91 -6.05 20.27 2.12 -130.15 -13.40 -2.04 -55.45 -6.76 -214.95 -5.99 -96.57 -4.81 -6.08 -6.08 -130.70 -2.24 -12.57 8.13 -134.80 8.13 -4.81 -41.36 -0.00 -129.90 -4.46 16.26 -7.09 -134.80 -15.61 -5.30 -53.68 -9.66 -233.43 -16.88 -91.69 -4.36 -8.34 -8.48 -2.86 -1.47 -17.09 4.89 -3.92 -1.00 -4.58 -3.15 1.51 -1.00 1.58 -4.01 -9.21 -4.65 -2.21 -3.26 1.77 -2.89 -18.48 -10.89 4.88 CaSO4 CaCO3 CaCO3 CH4 CO2 CaMg(CO3)2 Fe(OH)3 FeS FeOOH CaSO4:2H2O H2 H2O H2S NaCl Fe2O3 KFe3(SO4)2(OH)6 FeS FeSO4:7H2O N2 NH3 O2 FeS2 FeCO3 S Notes: (1) Notice that the mixed water is highly supersaturated with respect to both aragonite and calcite. This is not too much of a surprise considering that the water has such high alkalinities and Ca concentrations in the water. (2) Also that there are many more mineral phases that have saturation indices that have been calculated because of the higher concentrations in all of the water. IV. Batch Reaction Modeling: How do these water react with solid phases? What happens if the water comes into contact with a solid material (e.g. some mineral or rock) and reaches equilibrium with that material. A. Some questions are: (1) How does the composition of the water change once it reaches equilibrium with the mineral phase? (2) How much of the mineral dissolves 9 B. Procedure: (1) Go back to the “input” tab on the lower left window. (2) Make sure that “end” is highlighted and click on the “Equilibrium Phases” button, which looks like a double headed arrow. This button will open a window listing various mineral phases at the top of the window. (3) Chose the mineral phase or phases that you want to estimate how much equilibrates with the water by clicking on the radio buttons. For this example, we’ll react the water with calcite. You can set the saturation index you want to achieve or leave it at the default of 0 (e.g. equilibrium). Also, you can choose how much material you want to have available for the reaction calculations. The default is 10 moles, which for most minerals, except for highly soluble ones, is in excess of the amount that can dissolve to reach equilibrium. (4) Put the cursor in the right window and click “Run…”. C. Results Because of the order of input solutions and the mixing between the two solutions, the mineral phase is reacted with the mixed solution. If you want to react a solid with one of the primary solutions, the reaction button would have to be put below the composition of the water. Alternatively, it is possible to use the “Save” button, which is a left pointing curved green arrow, to save the compositions of one of the water and use it in a second simulation. (1) Now the upper part of the output file will show the “Reaction Step”, the mixture used, phase assemblages that are reacted with the solution, in this case it is calcite, the solution composition, and a description of the solution: Reaction step 1. Using mix 1. Using pure phase assemblage 1. Mixture 1. 4.000e+000 Solution 1 1.000e+000 Solution 2 -------------------------------Phase assemblage-------------------------------Phase Calcite SI log IAP 0.00 -8.48 log KT Initial -8.48 1.000e+001 Moles in assemblage Final Delta 1.000e+001 9.685e-004 10 -----------------------------Solution composition-----------------------------Elements C Ca Cl Fe K Mg N Na S Molality Moles 5.043e-003 1.484e-003 4.798e-004 1.577e-006 3.070e-005 5.714e-004 8.860e-005 3.256e-004 1.517e-004 2.522e-002 7.419e-003 2.399e-003 7.884e-006 1.535e-004 2.857e-003 4.430e-004 1.628e-003 7.585e-004 ----------------------------Description of solution---------------------------pH pe = = 7.240 11.882 Charge balance Adjusted to redox equilibrium Activity of water Ionic strength Mass of water (kg) Total alkalinity (eq/kg) Total CO2 (mol/kg) Temperature (deg C) Electrical balance (eq) Percent error, 100*(Cat-|An|)/(Cat+|An|) Iterations Total H Total O = 1.000 = 6.867e-003 = 5.000e+000 = 4.530e-003 = 5.043e-003 = 25.000 = -4.665e-003 = -9.74 = 28 = 5.550865e+002 = 2.776094e+002 (2) Again the program calculates the distribution of species. Here are the results are shown for C and Ca concentration. As you will see, the concentration of Ca has decreased from 1.682 x 10-3 molar to 1.484 x 10-3 molar. This decrease makes sense because the original mixed water was supersaturated with respect to calcite and could not dissolve calcite, and will only precipitate calcite, thereby reducing the Ca concentration. ----------------------------Distribution of species---------------------------Species Molality Activity Log Molality Log Activity Log Gamma OHH+ H2O C(-4) CH4 C(4) HCO3CO2 CaHCO3+ MgHCO3+ CaCO3 CO3-2 MgCO3 NaHCO3 NaCO3- 1.899e-007 6.226e-008 5.551e+001 0.000e+000 0.000e+000 5.043e-003 4.428e-003 5.260e-004 5.649e-005 2.014e-005 5.556e-006 4.650e-006 1.224e-006 6.804e-007 2.006e-008 1.738e-007 5.759e-008 9.999e-001 -6.721 -7.206 1.744 -6.760 -7.240 -0.000 -0.039 -0.034 0.000 0.000e+000 -131.861 -131.861 0.001 4.069e-003 5.269e-004 5.190e-005 1.845e-005 5.565e-006 3.313e-006 1.226e-006 6.815e-007 1.838e-008 -2.354 -3.279 -4.248 -4.696 -5.255 -5.333 -5.912 -6.167 -7.698 -2.391 -3.278 -4.285 -4.734 -5.255 -5.480 -5.912 -6.167 -7.736 -0.037 0.001 -0.037 -0.038 0.001 -0.147 0.001 0.001 -0.038 11 FeHCO3+ FeCO3 Ca Ca+2 CaHCO3+ CaSO4 CaCO3 CaOH+ CaHSO4+ 4.438e-015 7.932e-016 1.484e-003 1.404e-003 5.649e-005 1.777e-005 5.556e-006 3.144e-009 6.551e-012 4.067e-015 7.945e-016 -14.353 -15.101 -14.391 -15.100 -0.038 0.001 9.998e-004 5.190e-005 1.779e-005 5.565e-006 2.881e-009 6.003e-012 -2.853 -4.248 -4.750 -5.255 -8.503 -11.184 -3.000 -4.285 -4.750 -5.255 -8.540 -11.222 -0.147 -0.037 0.001 0.001 -0.038 -0.038 (3) There is also a description of which mineral phases are in equilibrium with the solution: ------------------------------Saturation indices------------------------------Phase Anhydrite Aragonite Calcite CH4(g) CO2(g) Dolomite Fe(OH)3(a) FeS(ppt) Goethite Gypsum H2(g) H2O(g) H2S(g) Halite Hematite Jarosite-K Mackinawite Melanterite N2(g) NH3(g) O2(g) Pyrite Siderite Sulfur SI log IAP -2.69 -0.14 0.00 -129.00 -1.81 -0.28 1.69 -133.46 7.58 -2.47 -38.24 -1.51 -129.91 -8.47 17.17 -5.42 -132.72 -15.84 -3.54 -56.24 -6.70 -218.50 -8.59 -97.55 -7.05 -8.48 -8.48 -131.86 -3.28 -17.37 6.58 -137.37 6.58 -7.05 -41.39 -0.00 -130.91 -6.88 13.16 -14.63 -137.37 -18.05 -6.80 -54.47 -9.59 -236.98 -19.48 -92.67 log KT -4.36 -8.34 -8.48 -2.86 -1.47 -17.09 4.89 -3.92 -1.00 -4.58 -3.15 1.51 -1.00 1.58 -4.01 -9.21 -4.65 -2.21 -3.26 1.77 -2.89 -18.48 -10.89 4.88 CaSO4 CaCO3 CaCO3 CH4 CO2 CaMg(CO3)2 Fe(OH)3 FeS FeOOH CaSO4:2H2O H2 H2O H2S NaCl Fe2O3 KFe3(SO4)2(OH)6 FeS FeSO4:7H2O N2 NH3 O2 FeS2 FeCO3 S As you can see, the calcite saturation is now at zero. This is because we told the program to react with calcite until the water reached saturation. Of interest is the fact that the decrease in the Ca concentration (as well as the alkalinity) causes the saturation state with respect to aragonite to decrease as well from 2.25 to -0.14. Note that the water is now undersaturated with respect to aragonite. If there is a mixture of aragonite and calcite, then aragonite will dissolve, increasing the calcium concentration, supersaturating the solution with respect to calcite and causing calcite to precipitate – the so called “aragonite-calcite transformation”. 12 V. Batch reaction modeling: Equilibrium with different atmospheric compositions and its influence on geochemical reactions? One of the most important controls on carbonate mineral dissolution is the concentration of PCO2 in the water and its conversion to carbonic acid (H2CO3). The PCO2 of the atmosphere is an important control on pH but other solutes can also change the pH which in turn controls the relative proportions of H2CO3, HCO3- and CO32-. The species influence the solubilities of various minerals. A. Some questions are: (1) How much will the saturation state of our water sample change if it is in equilibrium with atmospheric PCO2 with a partial pressure of 10-3.5, similar to earth’s atmosphere, or in a high PCO2 atmosphere of 10-1.5 for example that might occur in a cave. (2) How much calcite can these two different waters dissolve? B. Procedure: (1) Go back to the “input” tab on the lower left window. (2) Now we will start a new simulation. Highlight “Simulation 2” after the “end” button of Simulation 1. Add another “end” to Simulation 2. Open the file by clicking on the + symbol. (3) Highlight “solution 1” in Simulation 1 and cut and paste to simulation 2 by using control C and control V to move the data to directly above the end of simulation 2. Be sure to highlight “end” before pasting the data into the simulation. (4) Provide an amount of gas for the water to equilibrate with. Click on the small dark green gas cylinder and a menu will open allowing you to choose which gas phase to equilibrate with and the amount of gas to provide in the atmosphere. Note: The description of the gas includes the total pressure of the atmosphere that is equilibrating. The default is 1 atm, such as would be expected at the earth surface. Other variables that can be changed include the initial volume of gas available for equilibration. You can also change the reaction from fixed pressure, where the pressure doesn’t change regardless of the reaction, or a fixed volume, which can be consumed by the extent of the reaction. (5) Input the partial pressure of the CO2. Our first example will be a partial pressure of 10-3.5 atm. This needs to be input as a decimal fraction – or in this case 0.000316 atm. 13 (6) Click “Run…”, then open the “output” simulation tab and open the folder for the Reaction button. C. Results Looking at the “Description of the solution” give the following table: ----------------------------Description of solution---------------------------pH pe = = 7.471 11.506 Charge balance Adjusted to redox equilibrium Activity of water Ionic strength Mass of water (kg) Total alkalinity (eq/kg) Total CO2 (mol/kg) Temperature (deg C) Electrical balance (eq) Percent error, 100*(Cat-|An|)/(Cat+|An|) Iterations Total H Total O = 1.000 = 4.357e-003 = 1.000e+000 = 3.037e-003 = 3.236e-003 = 25.000 = -5.359e-004 = -8.86 = 20 = 1.110154e+002 = 5.551591e+001 And the saturation state provide the following table of data: ------------------------------Saturation indices------------------------------Phase Anhydrite Aragonite Calcite CH4(g) CO2(g) Dolomite Fe(OH)3(a) FeS(ppt) Goethite Gypsum H2(g) H2O(g) H2S(g) Halite Hematite Jarosite-K Mackinawite Melanterite N2(g) NH3(g) O2(g) Pyrite Siderite Sulfur SI log IAP -3.36 -0.14 0.01 -128.25 -2.21 -0.76 1.50 -133.42 7.39 -3.14 -37.96 -1.51 -129.83 -9.33 16.80 -7.88 -132.69 -16.96 -3.79 -55.93 -7.28 -218.68 -9.03 -97.76 -7.72 -8.47 -8.47 -131.11 -3.68 -17.85 6.39 -137.34 6.39 -7.72 -41.11 -0.00 -130.83 -7.75 12.79 -17.09 -137.34 -19.17 -7.05 -54.16 -10.17 -237.15 -19.92 -92.88 log KT -4.36 -8.34 -8.48 -2.86 -1.47 -17.09 4.89 -3.92 -1.00 -4.58 -3.15 1.51 -1.00 1.58 -4.01 -9.21 -4.65 -2.21 -3.26 1.77 -2.89 -18.48 -10.89 4.88 CaSO4 CaCO3 CaCO3 CH4 CO2 CaMg(CO3)2 Fe(OH)3 FeS FeOOH CaSO4:2H2O H2 H2O H2S NaCl Fe2O3 KFe3(SO4)2(OH)6 FeS FeSO4:7H2O N2 NH3 O2 FeS2 FeCO3 S 14 If you compare these results with the previous calculation for solution 1, you’ll see some subtle changes. In particular, the total CO2 concentrations have risen from 3.236 x 10-3 to 3.223 x 10-3 molar. This reflects the addition of CO2 from the atmosphere. This change in the concentration of CO2 in the water has also reduced the pH from 7.5 to 7.471 and the change in the pH has decreased the saturation state of the carbonate minerals; aragonite has decreased from -0.11 to -0.14 and calcite has decreased from 0.01 to -0.04. These are very small changes because the water presumably was originally near saturation with atmospheric PCO2 and thus hasn’t changed much in its composition. What happens if there is higher PCO2 in the atmosphere than the water is in equilibrium with, for example, within a cave? Additional atmospheric gases could be H2S, which can also be modeled. Now let’s run a revised model with water number 1 which reaches equilibrium with a PCO2 of 10-3.5 atm (0.000316 atm) and PH2S of 10-5.4atm (about 4 ppm or 0.000004). D. Procedure: (1) Go back to the “input” tab on the lower left window. (2) We will start a new simulation, which will be simulation number 3. Highlight “Simulation 3” after the “end” button of Simulation 2. Add another “end” to Simulation 3. Open the file by clicking on the + symbol. (3) Highlight “solution 1” in Simulation 1 and cut and paste to simulation 3 by using control C and control V to move the data to directly above the end of simulation 2. Be sure to highlight “end” before pasting the data into the simulation. (4) Provide an amount of gas for the water to equilibrate with. Click on the small dark green gas cylinder and a menu will open allowing you to choose which gas phase to equilibrate with and the amount of gas to provide in the atmosphere. (5) Input the partial pressure of the H2S with a value of 0.000004 atm. (6) Click “Run…”, then open the “output” simulation tab and open the folder for the Reaction button. E. Results We get the following table for the description of the solute. 15 ----------------------------Description of solution---------------------------pH pe = = 7.471 11.466 Charge balance Adjusted to redox equilibrium Activity of water Ionic strength Mass of water (kg) Total alkalinity (eq/kg) Total CO2 (mol/kg) Temperature (deg C) Electrical balance (eq) Percent error, 100*(Cat-|An|)/(Cat+|An|) Iterations Total H Total O = 1.000 = 4.357e-003 = 1.000e+000 = 3.037e-003 = 3.236e-003 = 25.000 = -5.359e-004 = -8.86 = 20 = 1.110154e+002 = 5.551591e+001 As you can see the major change in the water composition is that it now has a pe of 11.466, where the pe of the original water was 4. The reason the pe increases is that the program calculates pe based on concentrations of species in the redox couple. This change in the redox conditions changes the concentration of redox sensitive species. For example, the NO3- concentration decreased from 2.142 x 10-5 to 2.098 x 10-5. This change came about because of reduction of NO3- to NO2- and N2 gas. All of these species are now present in the water although they were absent before reaction with H2S. Another common redox couple is the reduction of SO42- and H2S, but in this case the added amount of sulfur in the system has now increased the SO42concentrations from 2.893 x 10-5 to 2.907 x 10-5. The total solubility of various solutes is listed in the table below: ------------------------------Saturation indices------------------------------Phase Anhydrite Aragonite Calcite CH4(g) CO2(g) Dolomite Fe(OH)3(a) FeS(ppt) Goethite Gypsum H2(g) H2O(g) H2S(g) Halite Hematite Jarosite-K Mackinawite Melanterite N2(g) NH3(g) O2(g) Pyrite SI log IAP -3.36 -0.14 0.01 -127.93 -2.21 -0.76 1.50 -133.06 7.39 -3.14 -37.87 -1.51 -129.51 -9.33 16.80 -7.88 -132.32 -16.91 -3.40 -55.62 -7.44 -218.07 -7.72 -8.47 -8.47 -130.79 -3.68 -17.85 6.39 -136.97 6.39 -7.72 -41.02 -0.00 -130.51 -7.75 12.79 -17.09 -136.97 -19.12 -6.66 -53.85 -10.33 -236.55 log KT -4.36 -8.34 -8.48 -2.86 -1.47 -17.09 4.89 -3.92 -1.00 -4.58 -3.15 1.51 -1.00 1.58 -4.01 -9.21 -4.65 -2.21 -3.26 1.77 -2.89 -18.48 CaSO4 CaCO3 CaCO3 CH4 CO2 CaMg(CO3)2 Fe(OH)3 FeS FeOOH CaSO4:2H2O H2 H2O H2S NaCl Fe2O3 KFe3(SO4)2(OH)6 FeS FeSO4:7H2O N2 NH3 O2 FeS2 16 Siderite Sulfur -8.99 -97.52 -19.88 -92.63 -10.89 4.88 FeCO3 S For a final simulation, we’ll run a revised model with water number 1 which reaches equilibrium with a PCO2 of 10-1.5 atm (0.0316 atm) and compare the values with the first time with a PCO2 of 10-3.5. F. Procedure: (1) Go back to the “input” tab on the lower left window. (2) If we don’t care about the results from the initial simulation with the H2S, we can modify “Simulation 3” by clicking off the concentration on the radio button for H2S, and then modifying the PCO2 to a value of 0.0316. (3) Run this simulation. G. Results We get the following table for the description of the solute. You can see that the solution is now considerably different that just the natural water composition or the composition of the water after it had equilibrated with atmospheric PCO2. For example, now the pH of the water is much lower, 6.6 rather than 7.5 for the original water. ----------------------------Description of solution---------------------------pH pe = = 6.628 12.518 Charge balance Adjusted to redox equilibrium Activity of water Ionic strength Mass of water (kg) Total alkalinity (eq/kg) Total CO2 (mol/kg) Temperature (deg C) Electrical balance (eq) Percent error, 100*(Cat-|An|)/(Cat+|An|) Iterations Total H Total O = 1.000 = 4.368e-003 = 1.000e+000 = 3.037e-003 = 4.514e-003 = 25.000 = -5.359e-004 = -8.83 = 16 = 1.110154e+002 = 5.551847e+001 The total solubility of various solutes is listed in the table below: 17 ------------------------------Saturation indices------------------------------Phase Anhydrite Aragonite Calcite CH4(g) CO2(g) Dolomite Fe(OH)3(a) FeS(ppt) Goethite Gypsum H2(g) H2O(g) H2S(g) Halite Hematite Jarosite-K Mackinawite Melanterite N2(g) NH3(g) O2(g) Pyrite Siderite Sulfur SI log IAP -3.36 -0.97 -0.83 -128.75 -1.36 -2.44 1.15 -133.61 7.04 -3.14 -38.29 -1.51 -129.50 -9.33 16.09 -6.42 -132.87 -15.79 -3.79 -56.44 -6.60 -218.19 -8.71 -97.08 -7.72 -9.31 -9.31 -131.61 -2.83 -19.53 6.04 -137.52 6.04 -7.72 -41.44 -0.00 -130.49 -7.75 12.08 -15.63 -137.52 -18.00 -7.05 -54.67 -9.50 -236.67 -19.60 -92.20 log KT -4.36 -8.34 -8.48 -2.86 -1.47 -17.09 4.89 -3.92 -1.00 -4.58 -3.15 1.51 -1.00 1.58 -4.01 -9.21 -4.65 -2.21 -3.26 1.77 -2.89 -18.48 -10.89 4.88 CaSO4 CaCO3 CaCO3 CH4 CO2 CaMg(CO3)2 Fe(OH)3 FeS FeOOH CaSO4:2H2O H2 H2O H2S NaCl Fe2O3 KFe3(SO4)2(OH)6 FeS FeSO4:7H2O N2 NH3 O2 FeS2 FeCO3 S As you can see the amount the water is undersaturated with respect to calcite and aragonite have increased by quite a lot to -0.97 for aragonite and -0.83 for calcite. VI. Downloading data If you are only interested in a specific result of the modeling calculations, it is possible to download those results into a file that can be opened in Excel or other similar programs, for example notepad etc. Downloading is possible through the selected_output button, which looks like a funnel with an equal sign, and is located on the “Printing and numerical methods” ribbon at the top of the page. If it is not displayed, right click on the ribbons at the top and you will be given the option of turning on and off the ribbons. Place your cursor at the end button and click the selected output button. This will provide a window with a series of tabs at the top. The General tab will be open when the window opens and will allow you to control options of several calculations such as pH, pe, charge balance, percent error etc. Each option has a drop down menu in which “true”, “false” or “as is” is selected. True indicates the option will be downloaded, false indicates it will not be printed. The other tabs in the selected output menu allow you to control which other results may be downloaded. The tabs include: Totals: Click on the radio button of elements for which you would like to download the total concentration of that element 18 Molalities and Activities: Will download the concentrations (in molality) and activities of various complexes and dissolved species Equilibrium Phases and Gases: Will download the amount of a solid phase that will dissolve or precipitate during a reaction, or the partial pressure of a gas which is in equilibrium with the water Saturation indices: Will download the saturation state of a water for which you have provided concentrations or by reaction with equilibrium phases VII. Running examples The help file (Parkhurst, D.L, and Appelo, C.A.J., 1999, User’s guide to PHREEQC (Version 2) USGS Water-Resources Investigations Report 99-4259) in Phreeqc is quite useful. You can access the file by clicking the help button in the menu. Much of the manual describes the program, data input, running the program etc, but at the end of the manual are 18 examples for each of the various simulations the program will run. The examples are easy to try and they give a good sense of what the program does and how you can input your own data. If you open one of the examples, you will see text describing the specific example, including the data (or simulation) that is to be used in the example. Following the introduction is a text file of the input parameters. The simplest method for trying one of the programs is to simply copy the text from the manual and paste it into the window on the right (not the workspace window). Once it is pasted into the window, you can open the Simulation file in the workspace window and you will see the data and simulation programming there. From there you can scroll through the output file and observe what calculations have been completed. In these examples, you will find two other applications that PHREEQ can do. These include: Transport modeling: This technique will calculate how migration of solutes will be transported through advection, dispersion, diffusion and with chemical reactions. Inverse modeling: Models the geochemical reactions and mixing between different waters that account for changes in composition along a flow path. 19