Option E past paper questions
Option E past paper questions
1. (N09) It is now widely accepted that the increased production of carbon dioxide is leading to global warming.
(a)
(b)
Describe how carbon dioxide acts as a greenhouse gas. [2]
Discuss the influence of increasing amounts of greenhouse gases on the environment. [3]
2. (N09) The biochemical oxygen demand (BOD) is a measure of water pollution.
(a) State what is meant by the term biochemical oxygen demand (BOD). [2]
(b) Identify the stage of sewage treatment which removes the substances responsible for high
BOD values and explain how this is done. [2]
(c) Describe how the addition of nitrates or phosphates to water can increase the BOD value
of a water sample. [2]
3. (N09) Identify two pollutants, other than carbon dioxide and carbon monoxide, emitted by car
exhausts and describe how each is produced. [3]
4. (N09) L andfill sites are used to dispose of about 90 % of the world’s domestic waste, but
incineration is being increasingly used in some countries.
(a) State one advantage of each method. [2]
Landfill:
Incineration:
(b) Suggest why some biodegradable plastics do not decompose in landfill sites. [1]
(c) High-level and low-level wastes are two types of radioactive waste. Compare the half-lives
and the methods of disposal of the two types of waste. [3]
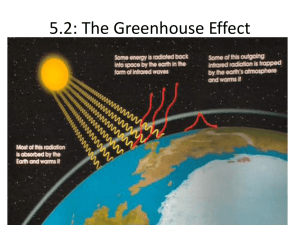
5. (M09) The greenhouse effect maintains the earth’s temperature, which makes the planet habitable.
However, over the last 100 years the average temperature of the earth has increased by almost
1 o C . Most climate scientists believe this warming is due to increased levels of greenhouse gases in
the atmosphere.
(a) Two of the major greenhouse gases in the atmosphere are methane and carbon dioxide.
State two other major greenhouse gases. [2]
(b) Discuss which two gases from the four gases in part (a) are the most significant for global
warming. [2]
(c) Discuss two effects of global warming. [2]
6. (M09) Water purity is often assessed by reference to its oxygen content.
(a) Outline the meaning of the term biochemical oxygen demand (BOD). [2]
(b) Describe how the dissolved oxygen concentration in a river would decrease if
(i) a car factory releases warm water into the river after using it for cooling. [1]
(ii) a farmer puts large quantities of a fertilizer on a field next to the river. [1]
(c) The Winkler method uses redox reactions to find the concentration of oxygen in water. 100
cm 3 of water was taken from a river and analysed using this method. The reactions taking
place are summarized below.
IB option E past paper questions
1
(i) State what happened to the O2 in step 1 in terms of electrons. [1]
(ii) State the change in oxidation number for manganese in step 2. [1]
(iii) 0.0002 moles of I – were formed in step 3. Calculate the amount, in moles, of oxygen, O2,
dissolved in water. [1]
(d) Due to the shortage of fresh water in many parts of the planet, scientists have designed ways to
convert sea water to fresh water. Outline how multi-stage distillation converts sea water to fresh
water. [3]
7. (M09) The ozone in the stratosphere protects us from harmful UV radiation. Above Australia there
is an area of decreased ozone concentration that has led to an increase in the incidence of some
skin cancers.
(a) Write equations for the natural formation of ozone. [2]
(b) Discuss the advantages and disadvantages of using hydrocarbons in place of CFCs.
[2]
8. (M09) The exhaust gases of automobiles contribute significantly to air pollution in cities.
(a) Outline how the pollutant gases nitrogen(II) oxide, NO, nitrogen(IV) oxide, NO2 and carbon
monoxide, CO, are formed as a result of the action of the internal combustion engine.
[3]
NO NO
2
CO
(b) One way to reduce automobile pollution is to control the fuel to air ratio. Discuss the impact of
increasing the fuel/air ratio on the concentrations of volatile organic compounds (VOC’s),
CO and NO in exhaust gases. [3]
9. (M09) Chlorofluorocarbons, CFCs, deplete the ozone layer.
(a) State the name of one source of CFCs. [1]
(b) In terms of their properties, suggest two reasons why CFCs continue to be used widely.
[2]
(c) State one advantage and one disadvantage of using tetrafluoromethane as an alternative to
CFCs. [2]
10. (M09)
(a) The following molecule is found in soil organic matter, SOM.
IB option E past paper questions
2
(i) State a main constituent of SOM. [1]
(ii) Explain, with reference to the structure above, how SOM can increase the soil quality, in terms
of the following. [3]
Provision of nutrients:
Water retention:
(b) Discuss how irrigation can cause soil degradation. [3]
(c) State the name of one source for each of the following organic soil pollutants. [2]
polyaromatic hydrocarbons (PAHs): organotin compounds:
11. (Specimen paper) The supply of sufficient drinking water continues to be a problem for the world.
One method used to provide drinking water from seawater is reverse osmosis, which uses a partially
permeable membrane.
(a) Outline the meanings of the terms osmosis and partially permeable membrane .
Osmosis
Partially permeable membrane
[2]
(b) Explain the technique of reverse osmosis used to produce drinking water from seawater.
[3]
12.
(spec) For each of the pollutants below, state one chemical method, different in each case, used to
reduce the amount entering the atmosphere. Write one relevant equation relating to the chemistry
behind the method.
(a) Carbon monoxide, CO [2]
(b) Nitrogen(II) oxide, NO [2]
(c) Sulfur(IV) oxide, SO
2
[2]
(d) Gasoline (petrol), C
8
H
18
[2]
13. (spec)
(a) Explain, including an equation, why rain falling in unpolluted air is acidic with a pH of about 6.
[2]
(b) Acid rain has a pH value less than 5.6. Explain, including an equation, how the burning of coal
can contribute to acid rain formation. [2]
(c) (i) Outline how acidic soil can damage the growth of trees. [1]
(ii) Write an equation for the reaction of acid rain on marble statues or limestone buildings. [1]
(d) Explain how the addition of calcium oxide to lakes neutralizes the effects of acid rain. [1]
14 (M10)
(a) Two primary air pollutants are carbon monoxide and oxides of nitrogen. State one man-made
source for each of these pollutants. [2]
carbon monoxide: oxides of nitrogen:
(b) Explain how a catalytic converter works and give the equation for the catalysed reaction between
carbon monoxide, CO, and nitrogen monoxide, NO. [3]
(c) State one other type of primary pollutant, other than carbon monoxide and oxides of nitrogen, that
IB option E past paper questions
3
can also be removed from the air by a catalytic converter. [1]
(d) Particulates may be produced by the burning of fossil fuels. Explain how small particulates can be
removed from the exhaust gases of coal-burning power stations before they enter the atmosphere.
[2]
15. (M10) The disposal of all types of waste is an increasing problem. One method of removing waste is
incineration.
(a) State one advantage and one disadvantage of incinerating waste. [2]
advantage: disadvantage:
(b) State the characteristics and sources of low-level nuclear waste. [2]
(c) The disposal of nuclear waste in the sea is now banned in many countries. Discuss one method of
storing high-level nuclear waste and two problems associated with it. [3]
16. (M10)
(a) Stratospheric ozone is in dynamic equilibrium with oxygen. Give the equations that describe the
formation of ozone from oxygen and its depletion in the stratosphere in the presence of ultraviolet
light. [2]
formation: depletion:
(b) Since the Montreal Protocol in 1987, the use of ozone-depleting CFCs has almost been phased
out. Discuss one advantage and two disadvantages of using hydrocarbons such as 2-
methylpropane as a replacement for CFCs. [3]
17. (M10) The greenhouse effect maintains the Earth’s average temperature at a habitable level. The components of the Earth’s atmosphere responsible for this effect are called greenhouse gases.
(a) Major greenhouse gases are water vapour and carbon dioxide. State two other greenhouse
gases. [2]
(b) Describe how greenhouse gases cause the greenhouse effect. [3]
(c) Discuss three possible implications of global warming on world food production. [3]
18. (M10)
The greenhouse effect maintains the Earth’s average temperature at a habitable level. The
components of the Earth’s atmosphere responsible for this effect are called greenhouse gases.
(a) Major greenhouse gases are water vapour and carbon dioxide. State two other greenhouse gases. [2]
(b) Describe how greenhouse gases cause the greenhouse effect. [3]
(c) Discuss three possible implications of global warming on world food production. [3]
19. (M01) Disposal of radioactive waste is a major ecological concern.
(a) State one source of low-level radioactive waste and one source of high-level radioactive waste. [2]
Low-level waste: High-level waste:
(b) Consider the following types of radioactive waste.
IB option E past paper questions
4
Identify which method can be used for the disposal of radioactive wastes A , B and C .
(i) Vitrification followed by long-term underground storage: [1]
(ii) Storage in a non-shielded container for two months followed by the disposal as
normal (non-radioactive) waste: [1]
(iii) Ion-exchange and adsorption on iron(II) hydroxide, storage in a shielded container for 50 years,
then mixing with concrete and shallow land burial: [1]
20. (M10) The ozone layer protects living organisms from dangerous UV radiation. In the Earth’s
stratosphere, ozone is photochemically formed from oxygen by the following two-step process.
(a) Ozone decomposition can proceed photochemically. Describe, using chemical equations, the two-
step mechanism of photochemical decomposition of ozone in the Earth’s stratosphere. [2]
Step 1: ....................................................................... Step 2:
(b) Ozone decomposition can also be catalysed by ozone-depleting substances such as
chlorofluorocarbons, CFCs. State two alternatives to CFCs. [1]
21. (M10)
Intensive farming changes the composition of soils and may lead to soil degradation. Common types of soil degradation include salinization , nutrient depletion and soil pollution .
Discuss two types of soil degradation. In your answer you should describe how each type of soil degradation occurs and suggest one negative effect on the environment. [4]
IB option E past paper questions
5
Mark scheme
1.
2.
3.
IB option E past paper questions
6
4.
5.
IB option E past paper questions
7
6.
7.
IB option E past paper questions
8
8.
9.
10.
IB option E past paper questions
9
11.
12.
13.
IB option E past paper questions
10
14.
IB option E past paper questions
11
15.
16.
17.
IB option E past paper questions
12
18.
19.
IB option E past paper questions
13
20.
IB option E past paper questions
14