Chapter 15
advertisement

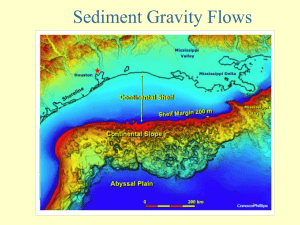
Chapter 17. Environmental Thermodynamics Problem 1. The definition of the octanol-water partition coefficient Prove that the octanol-water partition coefficient of a compound i can be expressed via the infinite dilution activity coefficients as (at 25 oC): iw, 17.25 K ow 0.151 o , i where iw , and io , are the infinite-dilution activity coefficients of the compound i in the aqueous and octanol phases, respectively. The following data are required: concentration of water in the organic phase Cw0=2.6 mol/l, xw0=0.268 mol/mol (organic phase), concentration of octanol in the aqueous phase Cow=4.5x10-3 mol/l, xow=0.703x10-4 mol/mol (aqueous phase). Hint: Write all concentrations in terms of number of moles via e.g. the equations Ci=ni/V and xi=ni/ntotal Problem 2. Concentration of pesticides in Amvrakikos Gulf (Greece) [Data from Voutsas and Tassios45] i. Calculate the distribution (ratio) of pesticides between biota and sediment in Amvrakikos Gulf and compare the result to the experimental values reported for two pesticides, given in table 17.8. Table 17.8. Experimental values for two pesticides. Pesticide Biota (Eels A. Anguila) Sediment (μg/Kg) (μg/Kg) Lindane 4.25 1.35 a-BHC 6.48 2.86 What do you observe? Does the distribution of pesticides between biota and sediment depend on the pesticide? ii. It has been found that the fraction of organic carbon in the sediment of Amvrakikos Gulf is 0.0375. Calculate again the distribution of pesticides between biota and sediment. What do you observe? Problem 3. PCBs in the aquatic organisms of the St. Lawrence River (USA, Sandler3) The concentration (mass fraction) of PCBs (polychlorinated biphenyls) in the St. Lawrence River in USA has been reported to be about 0.3.10-9. There are more than 100 different PCBs and a reasonable average value of their Kow is: log (Kow)=5.5. What is the concentration of PCBs in fish? 1 What do you observe if you compare the concentration of PCBs in water and in fish? How does the result compare to the experimental value measured for eels? (=7.9x10-6). Problem 4. Distribution of chemicals in aquatic organisms - The Bioconcentration Factor Insecticides in Fish The insecticide chlordane is found in lake-water at a concentration level approaching 560 g / l . If the BCF (in fish) is 14000 lt/kg, what is (in mg/kg) the concentration of the insecticide in fish? Problem 5. Distribution of benzo(a)pyrene in the environment of Southern Ontario in Canada (Adapted from Sandler3) It has been reported3 that the mass fraction of benzo(a)pyrene in the water in the southern part of the Canadian province of Ontario is 2.82.104 ng/m3. The vapor pressure of this compound is 2.13.10-5 Pa at T=298.15 K, its log(Kow)=6.04 and its value of the infinite dilution activity coefficient in water has been measured to 3.78.108. For this system, Sandler stated that Koc=0.615Kow (for soil) and Koc=0.5822Kow (for sediment). Calculate the concentration of benzo(a)pyrene in each of the (other) environmental compartments, i.e. the air, the soil, the sediment and the biota, with three different approaches: Method I : Using the reported value of log(Kow)=6.04 Method II: Using Kow calculated from the experimental value for the infinite dilution activity coefficient with the equation proposed by Sandler2,3: 17.26 log KOW ,i 0.486 0.8061log( iw, ) Method III: Assuming ideal liquid solution and Kow again calculated from the previous equation 17.26. Then, fill table 17.9 and compare your results to the experimental data. Comment on the accuracy of the approach. How different would the results be if you had chosen to use the Kow estimated from the UNIFAC-LLE model, which is log(Kow) (UNIFAC-LLE) = 7.3. How different would the results be if you would use the Koc-Kow relationships for soil and sediment mentioned in the chapter (equation 17.19). Table 17.9. Fill in the following table. Compartment C (I method) Water Air Soil Sediment Biota - C(II method) - C (III method) - 2 Reported [ng/m3 ] 2.82.104 1.3-7.1 1.5-7.5.108 (0.8-4).108 1.4.108 Problem 6. Selective separation of structurally similar compounds using supercritical fluid extraction (SCFE) SCFE is a method that can be used when mild separation conditions are desired or required and/or when separation of structurally-similar compounds e.g. isomers is needed. Such compounds may have appreciable (and different) solubility in supercritical fluids such as CO2, although particularly in the case of solids, use of co-solvents is often required to increase the solubility. The systems involved are often complex due to the nature of solutes (often polar/associating solids), the high pressures involved and the presence of co-solvents. For two typical saccharides (d-fructose and d-glucose), the solubilities in CO2 are of the order 10-5-10-7 (different experimental values in this range have been reported). Commercial applications will require use of co-solvents for increasing the solubility. What type of compounds should be used as co-solvents? Hint: Use the Walsh et al.42 concept and the Kamlet-Taft solvatochromic parameters. 3