Measurement Notes Word
advertisement

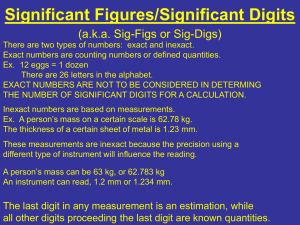
Notes: Measurement and Problem Solving Goal: The students will gain knowledge and understanding of the nature of physics and the importance of measurement and problem solving to the physicist. Prerequisite Objectives: Prior to beginning this unit the students should be able to: 1. Associate the units used for the measurement of mass, length and time with the instruments used for the measurements of these quantities. mass – kilograms – triple beam balance length – meter – meter stick, metric ruler time – seconds – stopwatch, clock, etc. 2. Use correctly the instruments for the measurement of mass, length and time in laboratory situations. See labs. 3. Use the SI (MKS) system of measurement. (pages 10-14) The International System of units – SI (Système International d’Unites – French name) – the meter, kilogram and second (MKS) are the three base units. During the French Revolution, the revolutionary government wanted a new measurement system that would have nothing to do with the monarchy. A commission was appointed and since 1800 the metric system has been official in France. Since then it has been in widespread use by scientists everywhere. Only a few countries do not use it officially at the present time, and most of them are thinking of changing. The system is popular because it is a decimal system and therefore easy to use. 4. Express numbers correctly in terms of scientific notation and use these values effectively in calculations involving the usual arithmetic operations. (pages 936-938) Scientific notation is based on exponential notation. In scientific notation, the numerical part of a measurement is expressed as a number between 1 and 10 multiplied by a whole-number power of 10. To write measurements using scientific notation, move the decimal point until only one non-zero digit remains on the left. Then count the number of places the decimal point was moved and use that number as the exponent of ten. To add or subtract in this system, all numbers must be changed so that the multiplier is the same power of ten. The numerical parts of the measurements are then added or subtracted, and the sum or difference is multiplied by the exponent of the original measurements. To multiply in this system, multiply the numerical parts and add the exponents algebraically. To divide in this system, divide the numerical parts and subtract the exponents. If the sum, difference, product, or quotient of the numerical parts is smaller than one or larger than ten, the accompanying exponents should be adjusted to bring 1 the portion of the answer containing the numerical part within the range of one to ten. 5. Use significant figures effectively in calculations involving the usual arithmetic operations. (pages 16-19) Significant figures are those digits in a measurement that are known with certainty plus the first digit that is uncertain. Rules for Determining How Many Significant Digits Are Present in a Number: 1. For numbers greater than 1 without a decimal shown move left to the first nonzero digit, it and all digits to the left of that are significant. 2. For numbers greater than 1 with a decimal shown all numbers are significant 3. For numbers less than 1 move right to the first non-zero digit, it and all digits to the right of that are significant One way of indicating the precision of a measurement is by means of significant figures. Significant figures should be used throughout the course. Rules for calculating with significant figures: 1. Addition or Subtraction: The final answer should have the same number of digits to the right of the decimal as the measurement with the smallest number of digits to the right of the decimal. Ex.: 123.567 78.9 63.25 372.644 638.361 Answer: 638.4 2. Multiplication or Division: The final answer has the same number of significant figures as the measurement having the smallest number of significant figures. Ex.: 10.6 12.34 = 130.804 Answer: 131 Note: Textbook answers will always show only the number of significant figures that the measurements justify. 6. Transpose a simple equation quickly and efficiently. (pages 938-939) Remember, whenever any operation is performed on one side of the equation, the same operation must also be done on the other side of the equation. Ex. ay/x = cb/s solve for x 7. Demonstrate Rules/Quiz.) safe Multiply by x: Multiply by s: Divide by cb: Rewrite: practices during laboratory ay = cbx/s ays = cbx ays/cb = x x = ays/cb investigations. Objectives: Upon completion of this unit, the students should be able to: 1. Distinguish physics from other areas of science. 2 (See Safety Physics is a science which describes and explains the interaction of matter and energy. Physicists gather information and organize it in words or in mathematical symbols. Some of the major areas of physics are: mechanics, thermodynamics, vibrations and wave phenomena, optics, electromagnetism, relativity and quantum mechanics. 2. Distinguish between base and derived units. A base unit is one that is not defined in terms of other units. The seven base physical quantities and their units are: length (meter), mass (kilogram), time (second), temperature (Kelvin), luminous intensity (candela), electric current (Ampere) and molecular quantity (mole). A derived unit is one that is defined in terms of base units. For example, the SI unit for area is the square meter and the SI unit for velocity is the meter per second. These are derived units. 3. Recognize that all measured quantities have uncertainties. All measurements are in error to some degree: 1. Human error – mistakes made in reading an instrument, or recording the results. To avoid this error, take repeated measurements to be certain they are consistent. Measurements must be made by looking at the device straight on. If they are not read straight on, an error due to parallax is possible. Parallax is the apparent shift in position of an object as it’s viewed from different angles. Ex.: reading the speedometer on a car from the passenger’s seat versus the driver’s seat. 2. Method error – measurements taken by different methods. To avoid this error, you should standardize the method of taking measurements. 3. Instrument error – equipment not in good working order. It is important to be careful with equipment. 4. External error – some equipment changes due to external causes. Ex.: length of a ruler changes with changes in temperature; electric measuring devices are affected by magnetic fields near them. 4. Distinguish between accuracy and precision. The uncertainty of a measurement can be expressed in terms of accuracy or precision. Accuracy of a measuring device depends upon how well the value obtained by using the instrument agrees with the accepted value. Thus, when a measurement to be made, the measuring device should first be checked for accuracy. This can be done by using the instrument to measure quantities whose values are known. The measured values are then compared to the known values. This is known as calibrating the instrument. Precision is the degree of exactness with which the measurement is made or stated. The precision of a measuring instrument is limited by the smallest division on its scale. Errors in measurements affect the accuracy of a measurement. But the precision is not affected since values are still stated in terms of the smallest division on the instrument. 3 The accuracy of measurements can be determined by comparing your results with the accepted value. The percentage error, or relative error, of a measured value can be found with the following equation: Relative Error = 5. Experimental - Accepted ×100% Accepted Demonstrate an understanding of the use of significant figures as a means of stating the precision of measured quantities. One way of indicating the precision of a measurement is by means of significant figures. Significant figures should be used throughout the course in laboratory investigations. 6. Use significant figures in measurements and calculations. See prerequisite objective 5. 7. Analyze experimental mathematically. data, expressing the relationship graphically and The independent or manipulated variable is any quantity that an experimenter can change at will. It is carefully varied during the experiment. It is placed in the first column of the data table. It is plotted horizontally on the x-axis, or abscissa. The dependent or responding variable is any quantity that changes in response to the experimenter’s manipulation of one or more independent variables. It is measured for each variation of the independent variable. It is placed in the second column of the data table. It is plotted vertically on the y-axis, or ordinate. A change in one of the independent variables causes a change in the dependent variable. Relationship di d i2 d 1/i d 1/i2 Change in Independent Variable i is doubled i is doubled i is doubled i is doubled Effect on Dependent Variable d is doubled d is quadrupled d is halved d is quartered The purpose of a scientific investigation is to discover relationships that may exist among the quantities measured. Physicists make their work easier by summarizing data in tables and graphs. Tables organize data and, usually, a clear trend can be seen in the data. A graph is a pictorial display of data. The shape of a graph may frequently reveal a relationship that is not apparent from a quick look at the data. Because a graph usually shows an obvious pattern, a smooth curve is drawn through the data points to make estimations for points without data. 4 Plotting data points that are directly proportional always results in the graph of a straight line. An equation expressing a direct (linear) proportion takes the form: d = ki, where i and d are variables and k is constant. d d do d do i i d= ki i d = ki + do d = -ki +do i2, If d is directly proportional to equation takes the form: d = ki2. the graph of the data will be a parabola. The do d d i i d = ki2 d= -ki2 + do Two quantities whose product is a constant are in inverse proportion. The graph of an inverse proportion is a hyperbola. The equation for an inverse proportion takes the form d = k/i. The graph of the inverse proportion d= k/i is symmetrical about the line d = i. The graph of the inverse square proportion is not symmetrical. It should also be noted that as i increases, d decreases much more rapidly in an inverse square relationship than it does in the simple inverse relationship. d d d=i d = k/i d=i i d = k/i2 5 i Sample Problem The data below was gathered in a laboratory using various batteries connected to a resistor and measuring the current flowing through the resistor. Voltage Current (Volts) (Amperes) 1.5 0.15 3.0 0.30 4.5 0.45 6.0 0.60 7.5 0.75 a. Which quantity should be plotted on the x-axis? b. Which quantity should be plotted on the y-axis? c. Sketch the graph of this data. Be sure to label each axis and include units. d. What type of function is shown by the graph? e. What is the slope of this graph? f. What are the units of the slope of this function? 8. Use dimensional analysis to check the validity of an equation. Units must be uniform when solving a problem. When writing an equation in physics, you must state the units as well as the numerical values. This helps you to keep consistent units in the equation. This also tells you if the equation is dimensionally correct (dimensional analysis). mass = density volume kg kg 3 m 3 m Ex.: velocity = distance time m ms s m ms s incorrect!!! kg = kg correct!! The factor label method of dimensional analysis is one way to convert between different units. You must know certain conversion factors as well as understanding the metric system prefixes. See the inside back cover of the textbook for the metric system prefixes and conversion factors. In addition to those you will need to know the conversion factors for time for example 60 seconds are in one minute or 365.25 days in one year. Sample: If you used 18 g m 2 of power how many Watts did you use? hr 3 18g· m2 1kg 60 s 60 s 60 s 60 min 60 min 60 min s3 1000g 1 min 1 min 1 min 1 hr 1 hr 1 hr 6 1 Watt 1 kgm2 s3 Sample Problem q1q2 , where F is measured r2 in chirps, q is measured in wogs, and r is measured in nerds. Using dimensional analysis, determine the units for k. Practice appropriate methods (graphical and algebraic) for the solution of problems growing out of laboratory exercises or problem assignments. In an alternative reality, scientists have found that F k 9. The method used to solve physics problems consists of a number of logical steps. These steps are described below. 1. Read the problem carefully and make sure that you know what is being asked and understand all the terms and symbols that are used in the problem. Write down all given data. 2. Write down the symbol for the physical quantity or quantities called for in the problem, together with the appropriate unit. 3. Write down the equation relating the known and unknown quantities of the problem. This is called the basic equation. In this step you will have to draw upon your understanding of the physical principle involved in the problem. It is helpful to draw a sketch of the problem and to label it with the given data. 4. Solve the basic equation for the unknown quantity in the problem, expressing this quantity in terms of those given in the problem. This is called the working equation. 5. Substitute the given data into the working equation. Be sure to use the proper units. 6. Perform the indicated mathematical operations with the units alone to make sure that the answer will be in the units called for in the problem. This process is called dimensional analysis. 7. Estimate the order of magnitude of the answer. 8. Perform the indicated mathematical operations with the numbers. 9. Review the entire problem and compare the answer with your estimate. Sample Problem Each cubic centimeter of aluminum has a mass of 2.6 grams. What is the mass of a cube that measures 2.0 cm along the edge? If the cube is then measured to have a mass of 13 g, what mass of the cube is hollow? Sample Problem According to the laws of planetary motion, planetary orbits can be described by the p2 following equation: k 3 , where k is a constant that is the same for all the sun’s d planets, p is the period of revolution (in earth years) for a specific planet, and d is its average distance (in km) from the sun. The average distance between the sun and the earth is 1.496 x 108 km. Find the value of k for the solar system. 7 Sample Problem Using the value of k found in the previous problem, find the average distance between the sun and Jupiter, if the period of revolution is equal to 11.86 earth years. Sample Problem Jupiter has a period (time for one revolution around the sun) of 11.86 years. If its mean radius of orbit is 7.78 x 1011 m, calculate the average speed, in km/h, of Jupiter around the sun. 2πR v= Formula: T where v is velocity, in km/h R is the radius, in km T is the period in hours Sample Problem A farmer must move six bales of hay, each of which has a mass of 50. kg, from the floor of the barn to the hayloft, which is 5.0 m above the floor. How much work must the farmer do to move all six bales? Formulas: F = mg W = Fd where F is the force, in Newtons (1 kg-m/s2) g is the acceleration due to gravity (9.8 m/s2) m is the mass in kilograms d is the distance in meters W is the work in Joules (1 N•m = 1 J) Reference: Holt Physics, pages 3-33; 936-939; 949-950 8