Dynamic Jahn-Teller Character of Manganese(III) Spin

1
Dynamic Jahn-Teller Character of Manganese(III) Spin-Crossover Complex
[Mn(taa)] (H
3 taa = tris(1-(2-azolyl)-2-azabuten-4-yl)amine)
Motohiro Nakano 1 and Gen-etsu Matsubayashi
Department of Molecular Chemistry & Frontier Research Center, Graduate
School of Engineering, Osaka University, Toyonaka, Osaka 560-0043, Japan
Takasuke Matsuo
Department of Chemistry & Research Center for Molecular Thermodynamics,
Graduate School of Science, Osaka University, Toyonaka, Osaka 560-0043, Japan
Abstract Paraelectric behavior due to a dynamic Jahn-Teller effect has been found in the high-spin (HS) phase above T c
= 48 K of the manganese(III) spin-crossover complex [Mn(taa)] (H
3 taa = tris(1-(2-azolyl)-2-azabuten-4-yl)amine). The dielectric constant obeys the
Curie-Weiss law with an asymptotic Curie temperature of 26 K, suggesting competition between a low-spin (LS) phase and a ferroelectric-ordered (FO) phase at low temperatures. A phase diagram based on a 4-state Ising-Potts model incorporating both a virtual cooperative Jahn-Teller transition (HS FO) and a spin-crossover transition (HS LS) is proposed to elucidate the interrelation of the HS, LS, and FO phases.
1. Introduction
2. Experimental
3. Results and Discussion
3-1. Raman Spectra of [Mn(taa)]
3-2. Dielectric Behavior of [Mn(taa)]
3-3. DFT-Optimized Structure of High-Spin [Mn(taa)] Species
3-4. Phase Diagram Based on a 4-State Ising-Potts Model
4. Conclusion
Acknowledgments
1 E-mail address: moto@ch.wani.osaka-u.ac.jp (Motohiro Nakano)
References
2
3
1. Introduction
Spin-crossover complexes contain octahedrally coordinate d 4 d 7 transition metal ions and have two nearly degenerate electron configurations with different spin multiplicities, i.e.
high-spin (HS) and low-spin (LS) states [1-5]. The ground state configuration of these complexes can be switched from one to the other by varying ligand-field strength. This bistability is usually accomplished by coupling with molecular deformations, especially metal-ligand stretching modes, and known to be controllable through external fields including temperature, pressure, magnetic field, and so on. In many theoretical models, only totally-symmetric breathing mode is taken into account as the interaction mode relevant to spin-crossover phenomena. However, lower-symmetry modes may play an important role when the orbital degeneracy is not fully quenched.
Such low-symmetry deformations are sometimes noted in experimental works
[6-10]. In this paper, a manganese(III) spin-crossover complex, where the anisotropic deformation induced by the Jahn-Teller effect provides a rich variety of phase behavior, is discussed based on a simple mean-field model.
The title compound [Mn(taa)] (H
3 taa = tris(1-(2-azolyl)-2-azabuten-4-yl)amine) is known to have an abrupt spin-crossover phase transition [11, 12]. The LS phase consisting of 3 T
1g
( t
2g
4 : S
= 1) molecules transforms into the HS phase consisting of 5 E g
( t
2g
3 e g
1 : S = 2) molecules at above T c
= 48 K. The crystal structure of the HS phase has cubic symmetry: space group I 4 3 d , lattice constant a = 2.031 nm, cell volume V =
N
N N
N N
Mn
N
N
3
E
3
A
C
3
C
3
+ LS
C
3
+ LS
+ JT
5
E
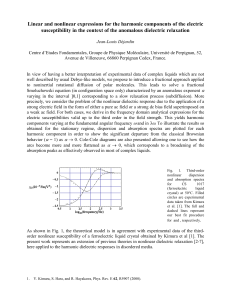
(a) (b)
Fig. 1. (a) Molecular structure of [Mn(taa)] and (b) splitting of ground multiplet under perturbations; C
3
twist (C
3
), spin-orbit coupling (LS), and E e Jahn-Teller distortion (JT).
4
8.377 nm 3 , and number of formula unit per unit cell Z = 16 [11]. The HS molecule in the cubic lattice has a C
3
axis penetrating the central Mn(III) ion and the non-ligating N(amino) atom of the taa 3 ligand (Fig. 1( a )). The coordination environment of the central Mn(III) ion is approximately octahedral with small twisting (9.2
) around the C
3
axis from the C
3v
symmetry.
2. Experimental
Polycrystalline [Mn(taa)] samples were prepared according to the literature method [11, 12] and tetrahedron-shaped single crystals were obtained by recrystallization from CH
2
Cl
2
/hexane solutions.
Variable-temperature Laser Raman spectra between 15-70 K were measured for a polycrystalline sample mounted in an Oxford CF1204 cryostat using a Jasco
NR-1800 Raman spectrophotometer.
The dielectric permittivity was measured on a compacted disk, 0.55 mm thick and 9.0 mm in diameter, with vacuum-deposited electrodes on the circular faces, using an HP 4284A precision LCR meter. The frequency and temperature ranges were 10 2 -10 6 Hz and 15-300 K, respectively. Temperature measurements were made by an Rh-Fe resistance thermometer fixed on the sample holder, which was calibrated against the ITS90. For the aid of thermal equilibration, a small amount of He gas (~10 Pa) was introduced in the sample chamber of the lab-made vacuum cryostat. Degradation of the sample by thermal cycling, e.g.
crack of the disk or strip-off of the Au electrodes, was negligible since no thermal hystereses of dielectric constants were detected.
3. Results and Discussion
3-1. Raman Spectra of [Mn(taa)]
The Raman spectrum of [Mn(taa)] recorded at 50 K is plotted in Fig. 2, together with a quantum-chemical simulation spectrum. The details of the theoretical method are presented in section 3-3. Although the simulation curve is not corrected with any scaling factors of vibrational frequencies, it is still useful for peak assignment. Temperature dependences of the Raman spectra are shown in Fig. 3. Some peaks showing remarkable intensity changes at around T c
are indicated by arrows. An important feature of these spectra is that most of
Raman-active vibrational modes show negligible frequency shifts across T c
, while
5 the intensitis vary due to probable site-symmetry changes are observed. This
6
7
8 behavior is in clear contrast to ordinary Fe spin-crossover complexes, where vibrational frequencies shift to lower as the sample changes from the LS to HS species [1-5, 13-18].
The shifts in vibrational frequencies between the LS and HS species are responsible for the major parts of the entropy changes S in spin transitions [13].
This has been repeatedly confirmed by infrared [13-15], Raman [14-17], and inelastic neutron scattering spectroscopies [18]. Since the observed entropy change 13.8 JK -1 mol -1 [12] in the present compound is not explained adequately solely by the entropy of the spin multiplicity ( R ln(5 / 3) = 4.25 JK -1 mol -1 ).
However, we cannot assign the extra entropy change to a vibrational origin, since the Raman spectra of the two phases differ only in intensities.
One of plausible candidates for the entropy source is a dynamic structural disorder in the HS phase, which should be settled down in the LS phase. The crystallographic data for [Mn(taa)] [11] provide a clue, i.e.
the presence of C
3
axis in the HS molecule. An Mn(III) ion in the 5 E state is a well-known Jahn-Teller ion [19]. Since the C
3
site-symmetry cannot lift the orbital degeneracy of the 5 E term (Fig. 1 (b) ), it is likely that the Mn ion is subjected to the E e Jahn-Teller effect, which gives rise to three energetically equivalent deformation structures.
The apparent C
3
symmetry should be observed in a time-averaged structure over three deformed structures.
3-2. Dielectric Behavior of [Mn(taa)]
The temperature dependence of dielectric constant at 1.0 kHz is plotted in
Fig. 4. The HS phase above the spin-crossover transition at T c
= 48 K shows a characteristic Curie-Weiss behavior,
∞
+ C / ( T – ), indicating the presence of dynamically disordering electric dipoles. The high-frequency dielectric constant is
∞
= 3.0, the Curie constant C = 91 K, and the asymptotic Curie temperature =
26 K. The positive value of suggests a ferroelectric interaction between reorienting dipoles in the HS phase. Such an interaction has a possibility of providing a ferroelectric phase at low temperatures, if the spin-crossover phase transition would not prevent the development of ferroelectric long-range order.
On the other hand, the Curie-Weiss behavior is fully suppressed in the LS phase below 48 K. It suggests that the complete C
3
-symmetry is recovered in the
LS molecule and the reorienting dipoles, which are required to guarantee the apparent C
3
-symmetry in the dynamically disordered HS phase, disappear. A slight growth of observed between 15-40 K arises from a small fraction of
9
Fig. 4. Temperature dependence of dielectric constant of [Mn(taa)] measured at 1.0 kHz. Full curve is the Curie-Weiss law, C / ( T - ), where C = 91 K and
= 26 K. thermally excited HS species. Analysis of this part assuming a temperature dependence of the form T -1 exp( eff
/ k
B
T ) for the permittivity gives the effective
LS-HS gap eff
/ k
B
= 340 K. This gap eff
is the energy difference between the
LS and HS states of a [Mn(taa)] molecule embedded in the LS molecules. Thus, it should differ from the LS-HS gap of an isolated molecule in vacuum by the van der Waals' interaction exerted by the surrounding LS molecules. This interaction is expected to enhance the energy gap because of mismatched molecular packing disturbing the uniform and equilibrium molecular array of the
LS phase.
A simple electrostatic model facilitates understanding the physical meaning of the Curie behavior of [Mn(taa)] in the HS phase. Electric polarization P produced by a number of reorienting molecular dipoles under a local field E loc obeys a simple Curie law,
P = ( N 2 / 3 k
B
T ) E loc
, where N stands for the number density of molecular dipoles. The local field E loc consists of the external field E ext
and the Lorentz field E
Lorentz
exerted from neighboring molecular dipoles,
E loc
= E ext
+ E
Lorentz
= E ext
+ P / (3
∞
0
), where
∞
is the high-frequency dielectric constant. From the definition of dielectric constant P = ( –
∞
)
0
E ext
, the Curie-Weiss law is provided:
=
∞
+ C / ( T - es
),
10 where C = N 2 / (3 k
B
0
), es
= C / (3
∞
), and
0
means the permittivity of vacuum.
Based on the experimental values, C = 91 K,
∞
= 3.0, and N = 1.91 nm -3 [11], the calculated value of the reorienting molecular dipole moment is = 1.25 D. The asymptotic Curie temperature in this electrostatic model is deduced to es
= C /
(3
∞
) 10 K, which is much smaller than the observed value, = 26 K. If we attribute the discrepancy between the calculated es
and the experimental solely to the van der Waals energy, we conclude that a neighboring pair of HS molecules prefers parallel deformation between them. Thus, the electrostatic contribution is about 40% of whole ferroelectric interaction between HS molecules, while the van der Waals interaction contributes up to 60 %.
3-3. DFT-Optimized Structure of High-Spin [Mn(taa)] Species
The geometry optimization of the HS [Mn(taa)] molecule was carried out by a hybrid DFT quantum chemical calculation using Gaussian98 program suite[20].
In the calculation, the ECP (effective core-potential) basis functions LANL2DZ were adopted for all atoms and a hybrid density functional UB3PW91 was used.
Since the C
3
-symmetry constraint is released, the optimized molecular structure is quite skewed (Fig. 5), so that three Mn-N(pyrrole) bond lengths are 1.9785,
1.9778, and 2.2032 Å, and three Mn-N(imino) bond lengths are 2.0642, 2.0365, and 2.3144 Å. Typical Jahn-Teller elongations along a coordination axis are found. Averaged bond lengths are 2.053 and 2.138 Å for Mn-N(pyrrole) and
Mn-N(imino) bonds, respectively, which correspond very well to the experimental values of 2.054 and 2.148 Å [11].
If the complex molecule maintains C
3
symmetry, it carries only longitudinal electric dipole parallel to the C
3
axis. Symmetry-lowering of the HS molecule produces the transverse component of electric dipole moment. Based on the optimized structure and the electron distribution, the longitudinal and transverse dipole moments are estimated to be 6.28 and 0.88 D, respectively. In the crystal lattice, reorientation of a whole molecule is prevented by dense packing, and only pseudo-rotation of the transverse moment is allowed. If we remember that the choice of the pseudo-C
3
axis, which affects separation of the longitudinal and transverse components, is not unique, the value obtained for the transverse moment may be regarded close to the experimental value of the reorienting dipole
1.25 D estimated from the dielectric constant. This good agreement verifies the idea of pseudo-rotating distortion dipoles attributable to the E e Jahn-Teller effect.
11
12
Upper 4 octants Lower 4 octants
Fig. 6. Molecular packing in [Mn(taa)] crystal. Coordination octahedra are colored according to four sublattices discriminated by mapping a body-diagonal of the unit cell to C
3
axes of member molecules. Darken side of each octahedron faces to the C
3
axis.
Since the 16 molecules in a unit cell are grouped into four sublattices in terms of their longitudinal moments, which align to four body-diagonals of the cubic unit cell, the Jahn-Teller distortions in the same direction generate non-collinear electric dipoles on different sublattices. The mutual relation of four sublattices is depicted in Fig. 6. Each octant of a unit cell contains two molecules belonging to a same sublattice, placed on the body-diagonal. Any two octants sharing a single corner are equivalent. Coordination axes (N-Mn-N) of a molecule are approximately parallel to the lattice vectors a , b , and c , and the tetragonal elongations of coordination octahedra by E e Jahn-Teller effect are regarded to take place along the lattice vectors. A transverse electric dipole is induced perpendicular to the molecular C
3
axis on a coplane of the C
3
and elongation axes. The lost collinearity suggests inequivalency of ferrodistortive and ferroelectric interactions between the HS molecules. For example, a ferrodistortive order with z -axis elongations makes electric polarizations of different sublattices cancel out each other resulting in an antiferroelectric order.
In Fig. 5 the spin-density isosurface of 0.005 e / a.u.
3 of [Mn(taa)] is also shown. Taking the Jahn-Teller elongation axis as z , the electron configuration is approximately described to be ( d yz
) 1 ( d zx
) 1 ( d xy
) 1 ( d z 2
) 1 . Most of the spin densities sit on t
2g
orbitals and around the z -axis, consistent with the electron configuration.
13
A small amount of spin densities appears on the pyrrole ring perpendicular to the z -axis, suggesting delocalization of t
2g
electrons over pyrrole orbitals sharing the nodal plane. It induces energy splitting between d yz
and d zx
orbitals and may cause rhombic zero-field splitting term in the spin Hamiltonian.
3-4. Phase Diagram Based on a 4-State Ising-Potts Model
A 4-state Ising-Potts model is applied to the [Mn(taa)] system to elucidate thermodynamic relations [21]. An [Mn(taa)] molecule is assumed to take four different microscopic states: state 0 is the LS state and states 1-3 are the HS states with the elongation axis parallel to x , y , and z , respectively. Interactions are assumed only between nearest neighbor molecules. Under a mean-field approximation [22], the internal energy of the [Mn(taa)] system is expressed in terms of populations i
( i = 0, 1, 2, 3) of four microscopic states,
U = (1 –
0
) + J
0
(
1
2 +
2
2 +
3
2 ) + 2 J
1
0
(1 –
0
), where (> 0) is the LS-HS gap, J
0
(< 0) is the Potts-type ferroelectric interaction between the HS species, and J
1
(> 0) is the Ising-type demixing interaction between HS and LS species [23]. The entropy S of the system consists of the contribution of spin-multiplicity and the entropy of mixing,
S / k
B
=
0 ln(2 S
LS
+ 1) + (1 –
0
)ln(2 S
HS
+ 1) -
3 i
0
i ln i
, where S
LS
= 1 and S
HS
= 2 are spin quantum numbers of the LS ( i = 0) and HS ( i =
1, 2, 3) states, respectively. By minimizing the free energy of the system F = U
– TS under a normalization condition
0
+
1
+
2
+
3
= 1, i.e.
solving
F / i
= 0, 2 F / i
j
> 0, ( i , j = 1, 2, 3), three stable solutions are obtained: an LS phase (
0
»
1
=
2
=
3
), an HS phase
(
0
«
1
=
2
=
3
), and a ferroelectric-ordered (FO) phase (
0
«
1
=
2
<
3
).
These three phases are shown in a T J
0
phase diagram (Fig. 7). The HS phase has the highest entropy and occupies high-temperature regions. Two distinct ordered phases, LS and FO, appear at lower temperatures, and the most stable phase observable near 0 K switches from one to the other at a critical value J
0
=
. The phase boundary between the HS and LS phases is given by
T c
( J
0
) = ( + J
0
/ 3) / k
B ln[3(2 S
HS
+ 1) / (2 S
LS
+ 1)].
Both spin-crossover transitions (HS LS, FO LS) are first order accompanied by definite jumps of populations, while the cooperative Jahn-Teller transition (HS
FO) is weak first-order (very close to a second-order transition). It suggests a possibility of observation of hidden cooperative Jahn-Teller transition (the broken
14
1.0
0.8
5
4
0.6
0.4
3
2
0.2
0.0
20 40
T / K
60
25
1
0
20 40
T / K
0.7
60
0.6
20
0.5
15
0.4
10
0.3
Fig. 7. Mean-field phase diagram of the 4-state Ising-Potts model ( / k
B
=
90 K, J
1
/ k
B
= 125 K). The high-spin phase (HS), the low-spin phase (LS), and the ferroelectric-ordered phase
(FO) are shown. The arrow line corresponds to J
0
/ k
B
= -36 K, appropriate to the [Mn(taa)] system.
5
0.2
0.1
0
20 40
T / K
60
0.0
20 40
T / K
60
Fig. 8. Thermodynamic quantities of the 4-state Ising-Potts model along the heating path (arrow line) in Fig. 7. line in Fig. 7) between the metastable HS and FO phases, if the HS phase could be supercooled enough below the spin-crossover transition temperature T c
by a rapid cooling.
The three adjustable parameters are determined, / k
B
= 90 K, J
0
/ k
B
= -36
K, and J
1
/ k
B
= 125 K, so as to reproduce the spin-crossover transition temperature T c
= 48 K, the virtual Jahn-Teller transition temperature T
JT
= 26
K, and the effective LS-HS gap in the LS phase eff
/ k
B
= 340 K. (Note eff
is approximated by + 2 J
1
in this mean-field model.) This choice of model parameters gives a phase sequence from LS to HS with increasing temperature, corresponding to the arrow path in Fig. 7. Temperature dependence of thermodynamic quantities (Fig. 8) is calculated along the path indicated by the arrow in Fig. 7, where the discontinuities arising from the first-order spin-crossover transition are recognized:
0
= 0.99, H = 0.64 kJ mol -1 , and S
15
= 13.3 J K -1 mol -1 . These theoretical values compare well with the experimental ones, H = 0.60 kJ mol -1 and S = 13.8 J K -1 mol -1 [12]. The good agreement supports the validity of the 4-state Ising-Potts model in the spin-crossover
[Mn(taa)] system.
Jahn-Teller effects are rarely adopted in theoretical treatments of spin-crossover phenomena, except Kambara's model for Fe(II) complexes [24] and Bersuker's for Fe(III) complexes [19, 25]. In the Kambara theory, abrupt spin-crossover transitions are given an FO LS character, while gradual transitions are of the HS LS type. However, experimental evidences of cooperative Jahn-Teller transitions (FO HS) have not yet been reported for
Fe(II) spin-crossover complexes. Kambara's theory ignores the spin-orbit interaction and appears to overestimate the Jahn-Teller coupling, producing an unphysical (for Fe(II) complexes) FO HS transition.
The most interesting possibility indicated by this phase diagram is that the
FO phase may be present as a metastable phase below T
JT
26 K, instead of the
HS phase above T
JT
. It suggests that physical properties of the metastable phase may change at T
JT
. Recently a photo-induced metastable phase of an Fe(II) spin-crossover complex [Fe(2-pic)
3
]Cl
2
•EtOH (2-pic = 2-aminomethylpyridine) was reported [26-28]. Its properties are remarkably different from those of the metastable HS phase quenched by rapid cooling. Lowered symmetry accompanying the Jahn-Teller deformation has been invoked to explain the difference. The situation has close similarity to the present compound. Thus,
[Mn(taa)] may be driven into a Jahn-Teller distorted FO phase by irradiation of light. The strong cooperativity indicated by the large value of J
1
/ k
B
= 125 K is a favorable factor [29], even though the HS LS transition has very small thermal hysteresis.
Recently high-field / high-frequency EPR spectra of [Mn(taa)] were recorded for a powder sample at 1017.6 GHz. Analysis of the spectra showed the presence of a rhombic zero-field splitting in the HS molecule [30], which is consistent with the symmetry lowering by E e Jahn-Teller distortions and the dynamic reorientation of deformation dipoles slower than the time scale of ~10 -12 s.
4. Conclusion
Spin-crossover phase transition of a manganese(III) complex [Mn(taa)] was studied by variable-temperature Laser Raman spectroscopy and it was found that
16 the vibrational contribution in the transition entropy is not dominant in contrast to the cases of ordinary iron spin-crossover systems. The discovery of a dynamic disorder in the HS phase by means of dielectric measurements provided an alternative entropy source to explain the thermally-induced spin-crossover transition. This dynamic disorder was attributed to the reorienting distortion dipoles accompanying the E e Jahn-Teller effect in HS manganese(III) ions.
Acknowledgments
The authors are grateful to Mr. Mitsuo Ohama at Osaka University for recording variable-temperature Raman spectra. This work was supported by a
Grant for Basic Scientific Research from the Sumitomo Foundation, a
Grant-in-Aid for Scientific Research from the Ministry of Education, Culture,
Sports, Science and Technology of Japan (MEXT), and a Strategic Research Base
Upbringing Special Coordination Fund for Promoting Science and Technology.
References
[1] O. Kahn, Molecular Magnetism (VCH Publishers, New York, 1993), Chap. 4.
[2] P. Gütlich, A. Hauser, and H. Spiering, Angew. Chem. Int. Ed. Engl.
33 , 2024
(1994).
[3] O. Kahn, Curr. Opinion Solid Stat. Mater. Sci.
1 , 547 (1996).
[4] A. Hauser, J. Jefti´c, H. Romstedt, R. Hinek, and H. Spiering, Coord. Chem.
Rev.
190-192 , 471 (1999).
[5] P. Gütlich, Y. Garcia, and H. A. Goodwin, Chem. Soc. Rev.
29 , 419 (2000).
[6] J. K. McCusker, A. L. Rheingold, and D. N. Hendrickson, Inorg. Chem.
35 ,
2100 (1996).
[7] M. Nakano, S. Okuno, G. Matsubayashi, W. Mori, and M. Katada, Mol. Cryst.
Liq. Cryst.
286 , 83 (1996).
[8] M. Nakano, A. Nakahama, S. Okuno, G. Matsubayashi, W. Mori, and M.
Katada, Mol. Cryst. Liq. Cryst.
376 , 399 (2002).
[9] J. M. Holland, J. A. McAllister, C. A. Kilner, M. Thornton-Pett, A. J.
Bridgeman, and M. A. Halcrow, J. Chem. Soc., Dalton Trans.
548 (2002).
[10] D. L. Reger, C. A. Little, and M. D. Smith, Inorg. Chem.
41 , 4453 (2002).
[11] P. G. Sim and E. Sinn, J. Am. Chem. Soc.
103 , 241 (1981).
[12] Y. Garcia, O. Kahn, J.-P. Ader, A. Buzdin, Y. Meurdesoif, and M. Guillot,
17
Phys. Lett. A 271 , 145 (2000).
[13] M. Sorai and S. Seki, J. Phys. Chem. Solids 35 , 555 (1974).
[14] M. Sorai, Bull. Chem. Soc. Jpn.
74 , 2223 (2001).
[15] G. Molnár, V. Niel, A. B. Gaspar, J.-A. Real, A. Zwick, A. Bousseksou, and J.
J. McGarvey, J. Phys. Chem. B 106 , 9701 (2002).
[16] A. Bousseksou, J. J. McGarvey, F. Varret, J. A. Real, J.-P. Tuchagues, A. C.
Dennis, and M. L. Boillot, Chem. Phys. Lett.
318 , 409 (2000).
[17] N. Suemura, M. Ohama, and S. Kaizaki, Chem. Commun.
1538 (2001).
[18] H. Paulsen, R. Benda, C. Herta, V. Schünemann, A. I. Chumakov, L. Duelund,
H. Winkler, H. Toftlund, and A. X. Trautwein, Phys. Rev. Lett.
86 , 1351 (2001).
[19] I. B. Bersuker, The Jahn-Teller Effect and Vibronic Interactions in Modern
Chemistry (Plenum, New York, 1983).
[20] Gaussian 98 , Revision A.11, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E.
Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, Jr.,
R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N.
Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B.
Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y.
Ayala, Q. Cui, K. Morokuma, N. Rega, P. Salvador, J. J. Dannenberg, D. K.
Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz,
A. G. Baboul, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R.
Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A.
Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong,
J. L. Andres, C. Gonzalez, M. Head-Gordon, E. S. Replogle, and J. A. Pople,
Gaussian, Inc., Pittsburgh PA (2001).
[21] R. M. Stratt and S. H. Adachi, J. Chem. Phys.
86 , 7156 (1987).
[22] M. Nakano, N. Fujita, G. Matsubayashi, and W. Mori, Mol. Cryst. Liq. Cryst.
379 , 365 (2002).
[23] C. P. Slichter and H. G. Drickamer, J. Chem. Phys.
56 , 2142 (1972).
[24] T. Kambara, J. Chem. Phys.
70 , 4199 (1979); ibid.
74 , 4557 (1981).
[25] N. Sasaki and T. Kambara, J. Chem. Phys.
74 , 3472 (1981).
[26] T. Tayagaki and K. Tanaka, Phys. Rev. Lett.
86 , 2886 (2001).
[27] T. Tayagaki, K. Tanaka, N. Yonemura, M. Shirai, and K.-I. Kan'no, Int. J.
Mod. Phys. B 15 , 3709 (2001).
[28] L. X. Chen, Z. Y. Wang, J. K. Burdett, P. A. Montano, and J. R. Norris, J.
Phys. Chem.
99 , 7958 (1995).
[29] X.-J. Liu, Y. Moritomo, A. Nakamura, T. Hirao, S. Toyozaki, and N. Kojima,
J. Phys. Soc. Jpn.
70 , 2521 (2001).
[30] S. Kimura, T. Otani, Y. Narumi, K. Kindo, M. Nakano, and G. Matsubayashi,
18
J. Phys. Soc. Jpn. Suppl.
in press; S. Kimura, private communication.