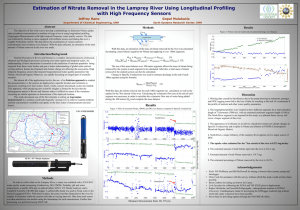
Assessment of Nitrate Concentrations in Local Water Bodies

Yakima WATERS Mini Lesson
Assessment of Nitrate Concentration in Local Water Bodies
Targets and Assessment
WA Science Standards Addressed:
9-12 INQA- Scientists generate and evaluate questions to investigate the natural world .
9-12 INQB - Scientific progress requires the use of various methods appropriate for answering different kinds of research questions , a thoughtful plan for gathering data needed to answer the question , and care in collecting, analyzing , and displaying the data.
9-12 INQC- Conclusions must be logical, based on evidence , and consistent with prior established knowledge.
Assessments:
Nitrate testing is just one component of a larger unit on water quality. A final lab report will be written detailing various pollutants levels, stream velocity, and aquatic invertebrate abundance (for example see “Rubric for Peer
Review”).
In the nitrate section in the final report students will be graded on their:
On line search of how nitrates gets into the water, what levels are safe in drinking water, and what nitrates do to the human body.
Graphical representation of nitrate levels throughout the body of water.
Lesson Parameters
Content Area: Biology, Chemistry, Geology
Overview: Investigating the nitrate levels of water bodies to understand pollutant accumulation and movement in watersheds.
Grade Level: 10th
Suggested Time: 1:45 minute period
Special Materials: Per group
One Nitrate HACH kit complete with nitrite 3 and nitrite 6 reagents, a HACH
DR/ 2000 spectrophotometer, and two test tubes with stoppers.
Various water samples (preferred within a single stream gradient so students can see accumulation of Nitrates downstream).
Learning Outcomes:
Knowledge: Nitrate testing can be used to evaluate the effects of human impacts on a water body.
Students will be able to recognize how pollution accumulates and migrates within a watershed if multiple water samples are taken from the same stream gradient.
Skill: In this lesson students gain exposure in: o Finding credible websites to access information o o o o o o
Following detailed instructions
Performing experiments
Handling and measuring chemicals
Graphing and Interpreting results
Sharing information with peers
Testing nitrate concentrations in local water
In writing the final report (next two lessons) students gain exposure in: o o o
Compiling information into a formal lab report
Creating and analyzing graphs using real data
Peer evaluation
o Disseminating information to interested parties
Science Concept Background:
Nitrate testing is part of a 6 day unit assessing pollution levels in Cowiche Canyon watershed
( http://www.cowichecanyon.org/ ). The canyon is located six and a half miles northwest of Yakima,
Washington. Water samples were taken from four sites along Cowiche stream starting at the base of the
Cascades and ending just before Cowiche Stream joins the Naches River.
Nitrates get into the water table via agricultural runoff from fertilizers, manure, crop residue, industrial waste, and human waste from septic tanks. In high agricultural areas such as the Yakima Valley, nitrate levels may be high. Nitrates interfere with hemoglobin in the blood which decreases the oxygen carrying capacity of the red blood cells in the body. Prolonged lack of oxygen in infants is called methemoglobinemia or “blue baby syndrome”. The Washington state standard for nitrate levels in drinking water cannot exceed 10 mg/l of water. Environmental effects include changing the composition and diversity of organisms due to algal blooms. Regular testing of waterways for nitrate pollution is necessary to preserve biodiversity, monitor general health of water body, and protect the health of the general public. http://www.doh.wa.gov/ehp/dw/Programs/nitrate.htm
http://www.epa.gov/ http://www.lenntech.com/periodic/elements/n.htm
Materials:
– All supplies can be purchased from: http://www.ohiopurewaterco.com/shop/customer/home.php?cat=342&gclid=CIyPye6slaUCFQoEbAodJEGIOQ o Water samples collected from local water body o 1 Nitrate Hatch Kit per group (3-4 students) containing:
Sufficient nitrate 3 and 6 reagents for number of water samples tested.
2 test tubes with rubber stoppers
1 nitrate color wheel
Procedure:
Begin class by having the students anonymously write the answers to the following questions on a piece of paper. They do not need any previous knowledge about pollutant just ask for common sense answers.
1.
How they think nitrates enter the water table.
2.
What high levels of nitrates can do to the human body?
3.
Why would it be important to regularly test water?
Paper should be balled up and tossed around the room for one minute to thoroughly mix. Next, going around the room students should read their answers to show their preconceived notions. Incorrect theories can be addressed at this time. Students should be given the following questions and asked to do an online search using credible web sites for the answers (see “Field Trip Follow up Nitrates and Phosphates”).
1.
How do Nitrates enter the water table? (hint: where do they come from?)
2.
What is the maximum amount of Nitrates drinking water can have for the Environmental
Protection Agency to consider it is still safe? (hint: how many milligrams of nitrates in 1 liter of water is safe to drink?)
3.
What will high levels of Nitrates do to the human body? (hint: describe diseases or effects it will have on our health - don’t just list them.)
Give a brief introduction to the HACH kits but allow the students to read the detailed instructions and decipher their contents in within their groups. A safety discussion should remind students not to eat or drink anything around their work bench, to use care in handling glass instruments, and the proper disposal of broken glass.
Instruct the students on proper disposal of waste solutions (into a hazard container at the front of the classroom) and all hands should be washed after handling the chemicals. A safety discussion should remind students that the reagents contain cadmium and although levels are extremely low care should be taken to wash hands thoroughly.
Finally, students will graph their results for each of the water samples (see the following example from “Field
Trip Follow up Nitrates and Phosphates”).
Please make a bar graph of Nitrate Levels at each field site.
Water sample #1 Water sample #2 Water sample #3 Water sample #4
Conclusion:
In final 15 minutes of class students will discuss their web findings with class and each group will plot their nitrate levels on a graph drawn on the front board to show variation among groups due to experimental error.
Discuss where errors could have occurred. Guide the discussion in the interpretation of the graph looking for trends.
Extension(s):
The students are required to write a formal lab report compiling previous lessons testing for fecal coliform, phosphates, water velocity, and aquatic invertebrate abundance in their water source. The students are responsible to compiling an introduction, methods, results of each water quality test, discussion, and literature cited. Their lab report will be reviewed by their peers and then submitted to the local land managers (in this case
Cowiche Canyon Conservancy).
Teaching Tips:
Work through the HACH kit so you will be able to field questions regarding the procedure. Find a local hazardous waste dispensary in your area.
Safety: Waste contains low levels of cadmium therefore, should not be dumped down the drain. Nitrate waste should be properly disposed of like other hazardous waste material or may be concentrated by evaporation in a safe place. ( http://www.water-research.net/Waterlibrary/watermanual/wqfieldmanual.pdf
)
Supplements:
See attached “Field Trip Follow up Nitrates and Phosphates” , “Rubric for Peer Review”, and “How to Write a
Field Report”.
Sources: http://www.water-research.net/Waterlibrary/watermanual/wqfieldmanual.pdf
http://www.ohiopurewaterco.com/shop/customer/home.php?cat=342&gclid=CIyPye6slaUCFQoEbAodJEGIOQ http://www.doh.wa.gov/ehp/dw/Programs/nitrate.htm
http://www.epa.gov/
http://www.lenntech.com/periodic/elements/n.htm
http://www.cowichecanyon.org/
Author: Melissa Reitz, Yakima WATERS Project, CWU, Fall 2010