C-KIT EXPRESSION IN NEUROBLASTOMA
advertisement

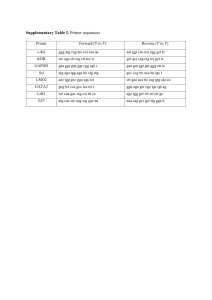
1 Ref ID: 103.1 c-KIT expression identifies a subset of aggressive neuroblastomas Uccini S.1, Mannarino O.1,2, McDowell H.P.3, Pauser U.4, Natali P.G.5, Altavista P.6, Andreano T.1, Boldrini R.2, Bosco S.1, Clerico A.1, Cozzi D.1, Donfrancesco A.2, Inserra A.2, Kokai G.3, Losty P.D.3, Nicotra M.R.5, Raschellà G.6, Vitali R.1,2,6, Dominici C.1,2 La Sapienza University1, Bambino Gesù Children’s Hospital2, Rome; RLC-NHS-Trust Alder Hey3, Liverpool; University of Kiel4, Kiel; Regina Elena Cancer Institute5; ENEA Research Center Casaccia6, Rome; UK, Germany & Italy. We showed recently that c-Kit is preferentially expressed in MYCN-amplified neuroblastomas and its signaling promotes in vitro neuroblastoma cell proliferation that can be selectively inhibited by STI571 (imatinib mesylate, Gleevec). This study aimed at further investigating in 168 neuroblastic tumors the clinico-biological characteristics of the subset that utilizes the SCF/c-Kit pathway and may be responsive to selective inhibitors. Expression of mRNA (by Northern blot) and protein (by immunohistochemistry) for c-Kit was detected in 22% and 13% of tumors, respectively; the latter was confined to neoplastic neuroblasts. Expression of mRNA and protein for SCF was documented in 31% and 28% of tumors, respectively, with 66% of the c-Kit-positive tumors also expressing SCF. Mutations in exon 11 of the c-kit gene were not found in the 9 c-Kit-positive and 9 c-Kit-negative tumors that were analysed. Expression of c-Kit correlated with advanced stage (3 and 4), MYCN amplification and 1p36 allelic loss (p<0.001); expression of SCF with adrenal primary (p<0.05), MYCN amplification and 1p36 allelic loss (p<0.001). Overall survival (OS) probability was 17% in c-Kitpositive cases vs. 68% in c-Kit-negative, 43% in SCF-positive cases vs. 78% in SCF-negative (p<0.001). Using Cox multiple regression analysis neither c-Kit nor SCF expression were independently associated with a shorter OS. The SCF/c-Kit pathway is expressed in a subset of particularly aggressive neuroblastomas for which selective therapeutic targeting is needed. (Partially supported by COFIN MIUR, CNR MIUR, AIRC, Italian Ministry of Health, ALTI) Presentation mode(s): ORAL-PRESENTATION – PC-PROJECTOR