Name APES Particulate Air Pollution Lab Background: Particulate
advertisement

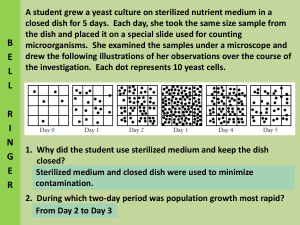
Name _____________________ APES Particulate Air Pollution Lab Background: Particulate matter is a term used to describe solid and liquid particles found suspended in the air. The particles have a wide variety of stationary and mobile sources and, therefore, a diverse set of physical and chemical properties. Particulate matter comprises a wide range of substances including road dust, wood smoke, fly ash, diesel soot, and sulfate aerosols. Most of these come from burning fossil fuels for transportation, power generation, and industrial boilers. Particles in the air range in size, from smoke and soot particle that are dark and large enough to see to particles so small an electron microscope is needed. The smaller particles are the greatest health hazard because they can penetrate more deeply into the respiratory tract. Since 1987 the EPA standard for particulate matter is PM-10, which include particle with a diameter of 10 micrometers or less (about 1/7 the width of a human hair). EPA's health-based national air quality standard for PM-10 as 150 µg/m3 (measured as a daily concentration). A microgram is a unit of mass and there are 1,000,000 micrograms in a gram. The major health problems from exposure to PM-10 are trouble with breathing, damage to lung tissue, cancer and early death. Those populations most affected by PM-10 are the very young, the elderly and those with lung disease and asthma. New scientific evident suggest that particle smaller than 2.5micrometers can cause serious health problems. The World Health Organization has done long-term studies that show the risk of premature death has a threshold of annual concentration of PM-2.5 or 10 g/m3. The EPA is reviewing possible changes in the existing PM-10 standard. Lab procedure: Step 1: Put your name on a piece of tape and then mark the bottom of 4 Petri dishes with your name and a number from 1 to 4. Using a quarter as a guide, draw a circle on 4 glass slides. Put one slide in each Petri dish. Step 2: Carefully smear a thin layer of petroleum jelly inside the circles you drew. Then, quickly put the lid on the Petri dish to keep the petroleum jelly clean until you are ready to use it. Step 3: Keep one Petri dish in the room with the cover on it. This will be your control. Choose 3 other locations in the school where you will be able to safely put your Petri dish. Place your Petri dish in the chosen spot and remove the lid. Answer part one questions. Step 4: After 48 hours, return to the spot, immediately cover the dish and return the dishes to the room. Step 5: Analyze your slides under a stereoscope (dissecting scope) or microscope. Count the actual number of particles you find caught in the petroleum jelly. Step 6: Complete the data grid and answer part two questions. Part one: Location of Petri dish: Hypothesize of types of particles to be collected Rank from clean (1) to dirty (5) Any factors to consider that may interfere with testing results Control Dish #1 Dish #2 Dish #3 Dish #4 1. Consider the Petri dish that you ranked as a “5,” indicating that you felt that area would result in the highest number of particles. Explain why you felt that way. 2. Consider the Petri dish that you ranked as a “2.” Explain why you felt this area would not have as much particulate matter. 3. In a PM-10 measurement, only particles that are 10 micrometers or less are considered. Why would larger particles not pose as much of a threat? 4. What is the current EPA standard for PM-10 for daily exposure? Part two: Location of Petri dish: Number of particles collected Rank from clean (1) to dirty (5) Hypothesize on the identity of the particles and their possible source. Control Dish #1 Dish #2 Dish #3 Dish #4 1. The diameter of a quarter gives an area of almost 5 cm2. Calculate the approximately number of particles that would be collected if your surface area was 1m 2. 2. In the previous problem, you calculated the particles in an area of 1m 2 . PM-10 is measured in micrograms per cubic meter (which is a measurement of volume). Explain how a technician could determine the mass in micrograms of particles in a cubic meter of air. 3. Do you think any of the particles you discovered would qualify to be in a PM-10 measurement? Why or why not? 4. Are the particles you collected considered to be pollution? Why or why not?