supplementary_material
advertisement

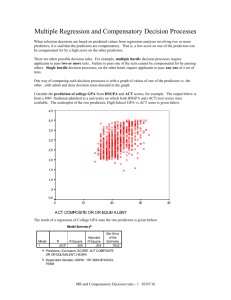
The metallization and superconductivity of dense hydrogen sulfide Supplementary Material Yinwei Li1*, Jian Hao1, Hanyu Liu2, Yanling Li1, and Yanming Ma2† 1 School of Physics and Electronic Engineering, Jiangsu Normal University, Xuzhou 221116, P. R. China 2 Department of Physics and Engineering Physics, University of Saskatchewan, Saskat chewan, Canada, S7N 5E2 3 State Key Laboratory of Superhard Materials, Jilin University, Changchun 130012, P. R. China Correspondence and requests for materials should be addressed to Y. L. and Y. Ma (yinwei_li@jsnu.edu.cn, mym@jlu.edu.cn). The supplementary material contains: The structural parameters of the predicted structures (Tab. SI1) Valence electron localization functions (ELF) of the predicted structures (Fig. SI1) The Phonon dispersion curves of the predicted structures (Fig. SI2) Band structures of the predicted structures (Fig. SI3) TABLE SI1. Calculated structural parameters of the predicted stable structures for H2S at selected pressures. Space group Pressure (GPa) Lattice parameters (Å) Atomic coordinates (fractional) P2/c 15 a = 5.6255 b = 3.4427 c = 6.9633 β =127.44° S (2e) S (2f) H1 (4a) H2 (4a) (0, 0.618, 0.25) (0.5, 0.996, 0.25) (0.155, 0.346, 0.225) (0.345, 0.273, 0.573) Pc 30 a = 5.0942 b = 3.1959 c = 5.3287 β =89.62° Pmc21 70 a = 4.6806 b = 2.9349 c = 4.9479 S1 (2a) S2 (2a) H1 (2a) H2 (2a) H3 (2a) H4 (2a) S1 (2a) S2 (2b) H1 (2b) H2 (2a) H3 (4c) (0.999, 0.192, 0.640) (0.494, 0.351, 0.342) (0.498, 0.062, 0.528) (0.977, 0.426, 0.857) (0.777, 0.052, 0.201) (0.271, 0.849, 0.739) (0, 0.179, 0.623) (0.5, 0.375, 0.355) (0.5, 0.048, 0.545) (0, 0.445, 0.858) (0.751, 0.119, 0.213) P-1 90 S1 (2i) H1 (2i) H2 (1g) H3 (1f) (0.308, 0.183, 0.245) (0.241, 0.415, 0.837) (0.000, 0.500, 0.500) (0.500, 0.000, 0.500) Cmca 170 a = 2.7804 b = 2.7926 c = 4.2568 α = 101.72° β = 76.53° γ = 109.58° a = 2.9986 b = 4.3274 c = 7.6005 S1 (8f) (0.000, 0.371, 0.615) H1 (8f) (0.000, 0.372, 0.270) H2 (8f) (0.000, 0.600, 0.092) Fig. SI1. Valence electron localization function (ELF) in the planes containing S-H bonds of the P2/c structure at 15 GPa, (b) the Pc structure at 30 GPa and (c) the Pmc21 structure at 70 GPa. ELF[1] is a local electrons pair probability for identification of localized electronic groups, and a dimensionless, empirical function in 3-space that generates relatively large values ranging between 0.5 and 1.0 in regions that can be ascribed to bonding and nonbonding localized electrons and smaller values (less than 0.5) where one expects the electrons to be delocalized. Fig. SI2. The calculated phonon dispersions of H2S for experimental Phase III (Pbcm) at ambient pressure (a), the predicted P2/c at 15 GPa (b), Pc at 30 GPa (c) and Pmc21 at 70 GPa (d). No any imaginary phonons were found to confirm the structural stabilities of these phases. Fig. SI3. (a)-(e) show the electronic band structures of H2S for the experimental Phase III (Pbcm) at ambient pressure, the predicted P2/c at 15 GPa, Pc at 40 GPa, Pmc21 at 70GPa, and P-1 at 120 GPa, respectively. The band gaps in (a e) have been revised by performing HSE06 [2, 3] hybrid functional calculations as implanted in the VASP code. References [1] A. D. Becke, and K. E. Edgecombe, J. Chem. Phys. 92, 5397 (1990). [2] A. V. Krukau, O. A. Vydrov, A. F. Izmaylov, and G. E. Scuseria, J. Chem. Phys. 125, 224106 (2006). [3] J. Heyd, G. E. Scuseria, and M. Ernzerhof, J. Chem. Phys. 118, 8207 (2003).