Complex
advertisement

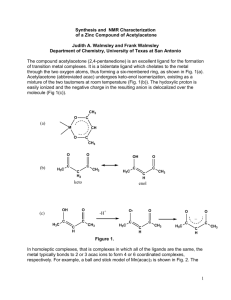
Metal Acetylacetonate Complexes Laboratory report for Chemistry 261 John Stephenson (410-1306) March 6, 2002 Introduction Acetylacetone, properly called 2,4-pentanedione is a -diketone that readily deprotonates to both carbonyls and then rearranges as it delocalises the electrons across the more electronegative oxygen atoms as shown in Scheme 1. This ketone is thus able to form a relatively weak acid in aqueous solution. As this happen in aqueous solution, water is an essential solvent and proton acceptor. Scheme 1: Formation of the acac anion from acetylacetone H H2 H2O CH3 C H3C CH3 C H3C H3O+ + O O O O H CH3 H3C O - O The acetylacetone anion that forms is then further able to act as a ligand to complex metal ions. The bonding happens as the two oxygen atoms in the ketone anion form a stable six-membered ring with the metal. The compounds that are typically formed are relatively insoluble in water and are of the formula M(acac)x from Mx+, where acac is a short hand notation for the acetylacetone ligand. The two metal acac complexes that were prepared in this laboratory by the reaction of Al3+ and Cu2+ with acetylacetone are shown in Scheme 2a and 2b. In the case of Al(acac)3 the aluminum cation forms an octahedral complex with acac with sp3d2 hybridization. With Cu(acac)2 the copper cation forms a tetrahedral complex with sp3 hybridization. In acac, the carbons in the C=O bonds and in the central carbon to both carbonyls forms trigonal planar bonds, sp2 hybridization. The oxygen atoms each form sp3 hybridization. Finally, the extra lone pair of electrons are delocalised across the acac side of the six membered ring. Scheme 2a: Formation of the Al(acac)3 from Al3+ and acetylacetone anion O O 3+ Al + 3 - O O O Al O O O Scheme 2b: Formation of the Cu(acac)2 from Cu3+ and acetylacetone anion O 2+ Cu + 2 - O O O Cu O O Experimental Preparation of Al(acac)3 3mL of acetylacetone was dissolved in a flask with 40mL of distilled water, followed by 8mL of 5M aqueous ammonia. In a separate flask, 3g of Al2(SO4)3.18H2O was dissolved in 30mL of water. The acetylacetone solution was slowly added to the metal salt solution. A cream-colored precipitate formed right away as progressed to precipitate as the acetylacetone solution was added. The pH was checked and found to be slightly basic. The solution stood for approximately 15 minutes, the cream-colored product was filtered at the pump. The sample was transferred to a weighed vial, covered with paper towel and placed in a vacuum oven to dry for 20 minutes. Then an attempt was made to recrystallize the sample using DCM, but no crystals ever precipitated. A sample of Al(acac)3 was obtained from another group to do the analytical work on. Preparation of Cu(acac)2 2.5mL acetylacetone in 5mL of methanol was dissolved in a flask. In a separate flask, 2.2g of CuCl2.2H2O and 15mL of distilled water was added. The acetylacetone solution was slowly added to the metal salt solution with stirring. To the mixture, 3.4g of NaOAc in 8mL of water was slowly added. During the additions the mixture goes from light blue, then to light green to dark green, and finally a blue-gray precipitate forms. Then the mixture was heated to 80C for 5 minutes. The flask was cooled to room temperature, then in an ice-bath. The precipitate was filtered at the pump, sucked dry for 15 minutes then transferred to a weighed vial. The vial was covered with paper towel and placed in a vacuum oven to dry for 20 minutes. The product was recrystallized from hot methanol and the analytical work done on the sample. Analytical work Melting points of both the aluminum and copper samples were determined using sealed end glass capillary tubes with a Mel-Temp II melting point apparatus made by Laboratory Devices, USA. An Oakton Digital thermometer was used in place of a 400C mercury thermometer. Infrared spectra were prepared by weighing approximately 20mg of sample and 200mg of KBr weighed. The samples were intimately ground together with a polished ceramic mortar and pestle, pressed at 10 tonnes of pressure. The KBr disks were analyzed with a Bomem Hartmann & Braun MB-series infrared spectrophotometer with PC interface. UV-VIS spectra were obtained for both samples by preparing a solution of approximately 10-3 M concentration in dichloromethane. The samples were analyzed with a UV-VIS apparatus with PC interface by first calibrating with a cuvet of DCM to get a baseline, then running a cuvet filled with each of the solutions. Results Table 1: Physical data on acetylacetonate complexes Product Yield Melting point Physical Appearance Al(acac)3 4.01g Cream colored powder 186-88 9.58mmol 106% yield (**) Cu(acac)2 3.51g Blue-grey colored 238-40 13.4mmol powder 110% yield (*) (*) 2.1g CuCl2.2H2O used / (170.48g/mol) = 12.3mmol expected. Note that the yield exceeds this value, indicating that the sample still contained some H2O (**) 3.0g Al2(SO4)3.18H2O used / (666.5g/mol) * (2 mol acac/mol Al2(SO4)3 )= 9mmol expected. Note the yield exceeds the value, indicated that the sample still contained some H2O. Table 2: UV-Vis data on acetylacetonate complexes Compound Wavelength, (nm) Absorbance, A Cu(acac)2 240 2.81 264 2.83 318 3.06 654 0.127 Al(acac)3 240 2.60 266 2.72 312 2.97 UV-Vis, IR and NMR spectra is attached at the end of this document. Discussion The crude yields for the compounds were quite high. Table 1 shows the yields were both nearly quantitative (as the samples were quite dry after the vacuum oven drying). Ideally these samples should have been left longer in the oven (perhaps, overnight) to ensure they had no water in them so a true yield could be obtained. If the calculated yield is still above 100% then it could be concluded that the samples contained other impurities. Ammonia and sodium acetate have a critical function in the synthesis of the acac complexes. To form the acac salts, acetylacetone must first be deprotonated then complex with the metal ion. As acetylacetone is a weak acid, little of the acac anion is formed in neutral solution. With a proton acceptor such as a weak base, like sodium acetate or ammonium hydroxide this pushes the equilibrium to form the acac ligand to complex with the salts. Organic solvents such as dichloromethane and methanol are used because the acac complexes are negligibly soluble in water. Unfortunately, Al(ac)3 did not precipitate easily. Perhaps the Al(ac)3 could be dissolved in a relatively non-polar solvent such as acetone and then slowly crashed out by the slow addition of water. The UV-Vis spectra for both Al and Cu complexes share peaks around 240nm, 265nm and 314nm as can be seen in Table 2. However the observed color difference between the Al and Cu complex can be seen in the spectra. The copper complex has a broad absorption peaking at a wavelength of 654nm. Absorbance at 654nm means that the compound will preferentially absorb orange-red light, and reflect blue-green light, and so, complex appears a beautiful blue-grey hue. NMR samples for acetylacetone were gathered from the library. In both of the 1H spectra for acetylacetone and Al(acac)3 we see the two –CH3 groups at the end of the acac integrate for 6 protons around a chemical shift of 1.9. The –CH– group in the middle of the two carbonyls can be seen in both spectra around a chemical shift of 5.4. The acetylacetone also has a third peak for the proton more downfield around a chemical shift of 15.4 that the Al(acac)3 compound does not have. The missing proton is the one removed by the base in the preparation to form the complex. In both 13C spectra of the two compounds, Al(acac)3 and acetylacetone we see the peaks representing the carbon containing the 3 protons of the methyl group of the acac molecules at =25.0. Also seen is the carbonyl carbons at =191.1. References Housecroft and Sharpe, 2001. Inorganic Chemistry, Prentice-Hall: Harlow, England. p. 308-309. Wang, S. 2002. “Chem 261 Course Material” Queen’s University: Douglas Library XE5.J06 Wang, S. 2002. “Chem 261: Laboratory Material” p 10-11.