Combustion and Emissions (Teacher Notes)
advertisement

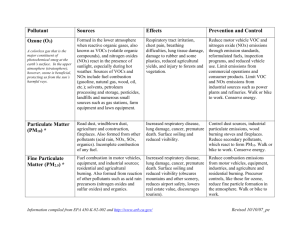
Combustion and Emissions (Lesson Plan) (Chemistry of combustion, emissions from automobiles, & primary and secondary pollutants) Suggested Grade Level 7-12 Standard Statements (Pennsylvania) 3.1.10.B Describe concepts of models as a way to predict and understand science and technology. 3.2.10.B Apply process knowledge and organize scientific and technological phenomena in varied ways. 3.2.12.B Evaluate experimental information for appropriateness and adherence to relevant science processes. 3.4.6.A Describe concepts about the structure and properties of matter. 3.4.10.A Explain concepts about the structure and properties of matter. 3.4.12.A Apply concepts about the structure and properties of matter. 3.5.12.C Analyze atmospheric energy transfers. 3.6.12.A Analyze biotechnologies that relate to propagating, growing, maintaining, adapting, treating and converting. 3.6.10.C Apply physical technologies of structural design, analysis and engineering, personnel relations, financial affairs, structural production, marketing, research and design to real world problems. 3.8.10.A Analyze the relationship between societal demands and scientific and technological enterprises. 3.8.12.A Synthesize and evaluate the interactions and constraints of science and technology on society. 3.8.10.C Evaluate possibilities, consequences, and impacts of scientific and technological solutions. 3.8.12.C Evaluate the consequences and impacts of scientific and technological solutions. 4.2.10.C Analyze how man-made systems have impacted the management and distribution of natural resources. 4.3.7.A Identify environmental health issues. 4.3.10.A Describe environmental health issues. 4.3.12.A Analyze the complexity of environmental health issues. 4.3.12.C Analyze the need for a healthy environment. 4.8.10.D Explain how the concept of supply and demand affects the environment. 4.9.10 A Explain why environmental laws and regulations are developed and enacted. 10.1.12.D Evaluate issues relating to the use/non-use of drugs. 10.2.9.E Explain the interrelationship between the environment and personal health. 10.2.6.E Analyze environmental factors that impact health. Content Objectives Students will know 1. The relationship between atoms, molecules, and elements. 2. The basic products and reactants of a combustion reaction. 3. Both the ideal and the real products of combustion in automobiles. 4. Specific effects of primary and secondary pollutants. Emissions Penn State University GREATT Project Lesson Plan 1 Process Objectives Students will be able to 1. Relate the products of combustion to population density and conditions of burning. 2. Relate combustion emissions to human and environmental health. 3. Identify the fuel in a given combustion system (candle, car, lighter, etc). Assessment Strategies 1. Evaluation of completed student handouts. 2. Group discussion of key ideas. 3. Evaluation of a reflective essay. 4. Evaluation of research project in Part 4. Materials Per group or per individual: Copies of student worksheets Per group or per entire class: Oil lamp Oil (do not use non-sooting oil) Candle (inside a jar) Coffee filters (bowl type for a drip coffee maker) Masking tape Vacuum cleaner with a hose attachment nozzle Procedures Part 1 (1, 50-min Class Period) 1. Decide in advance when you will want your students to complete the “Lung Attack” online tutorial referenced in the PowerPoint Presentation. 2. Present the PowerPoint presentation, “Vehicle Emissions,” noting that the presentation is broken into sections and can be tailored to the appropriate level of the class. Use the light blue action buttons to skip sections of the presentation that you do not feel are relevant to your class. (If you skip questions, adjust which questions in the student handout you want your students to answer.) 3. Introduce (or review) balancing chemical equations. Remind students that there should be the same number of each element on each side of the equation. 4. Help the students complete the section in their handout dealing with nitrogen oxides by reviewing the corresponding slides in the PowerPoint. Part 2 (20 min) 1. Prepare an oil lamp and light it. Adjust the wick so that the students can see black soot coming from the flame. Ask what is causing the soot. Explain that the flame is fuel rich, meaning that there is more fuel available than there is oxygen available. Therefore, there is incomplete combustion. 2. Cover the end of a vacuum cleaner nozzle with a coffee filter. Use a piece of masking tape to tape the ends of the filter to the nozzle. 3. Collect a sample of the “soot” from the flame by holding the nozzle about 6 inches above the flame for about 30 seconds. CAUTION: Be sure to hold the nozzle far enough above the flame so that you do not catch the filter on fire. Emissions Penn State University GREATT Project Lesson Plan 2 4. Adjust the flame size (by adjusting the lamp wick) so that there is no visible soot. You may want to clean the glass or have a spare glass cover available. Ask the students if they can see any soot coming from the flame. 5. Repeat the procedure for collecting the soot from the flame using the coffee filter and vacuum nozzle. There should be some soot on the filter for this second position of the lamp wick. Ask your students to compare the two filters and ask them why they look different. Basically, if you have a fuel lean system (i.e. more available oxygen), you obtain less soot. 6. Link this first experiment to the balanced combustion equation (this is a good place to link limiting reagents if in a chemistry classroom). Review ideal versus real emissions. 7. Place a candle in a jar, and light it so that the flame is below the lip of the jar. Ask the class what the fuel is for a candle flame. Ask them what component of air (nitrogen or oxygen) is necessary for combustion and how much nitrogen and oxygen are actually in the air (~80% and ~20%, respectively). 8. Cover the jar with a glass lid, such as a watch glass. Watch as the candle blows out. Ask the students why this occurs. Part 3 (Variable) 1. Have a class discussion to address any questions from Part 1 and Part 2 of the activity. 2. Allow the students to complete the essay described in Part 3 of their handout. Part 4 (Variable) 1. Assign the research project outlined in Part 4 of the student handout. Give students some time to figure out what topic they would be interested in researching further, and then ask for their topics. 2. Allow the students to present their research projects to the class. Emissions Penn State University GREATT Project Lesson Plan 3 Combustion and Emissions (Teacher Notes) (Chemistry of combustion, emissions from automobiles, & primary and secondary pollutants) General Lesson Notes What is the earth’s major source of energy? The sun. The sun’s energy creates wind for windmills, drives the water cycle for hydropower, and provides energy for plants to grow. How is that energy stored? The energy of the sun is captured by plants, which take carbon dioxide (CO2) from the air to build carbon-based macromolecules. There is energy stored in the new form of carbon that makes the plant. What happens when that energy is used? When the carbon-based fuel is burned it releases energy. This releases the carbon back to the air in the form of CO2. Part 1 Notes PowerPoint presentation. (This presentation pertains to Questions 1a to 1f in the Student Handout.) Navigate through portions of this show using the blue buttons provided. You may skip sections if they are not appropriate for your classroom. A review or introduction to balancing equations will be helpful for students. Student worksheets. If the emissions from an automobile were perfect, then these are the emissions that would be made. Only hydrocarbons and oxygen would produce carbon dioxide and water. For simplicity, explain to the students that the nitrogen plays no role in the reaction, but it is part of air (Air is made of 20-21% oxygen and 79-80% nitrogen) C8H16 + 12 O2 → 8 CO2 + 8 H2O The emissions from an automobile are not the result of perfect combustion. Real emissions include: • Carbon monoxide (CO) • Oxides of nitrogen (NO, NO2) • Carbon as soot or particulates • Unburned fuel (hydrocarbons) • Carbon dioxide (CO2) • Water (H2O) Lung Attack. (This explanation pertains to Questions 2a to 2d in the Student Handout.) This web-based activity (http://www.airinfonow.org/html/activities.html, requires Macromedia Flash) can be done as a class, in small groups, or individually. It demonstrates normal respiration, as well as the effects of carbon monoxide and particulate matter on the respiratory system. The Lung Attack animation takes about two minutes to run for each scenario. The basic respiration example is an excellent and engaging tutorial for students who are beginning to learn about lung function in biology or health class. It could also be used in conjunction with anti-smoking campaigns, since carbon monoxide and particulate matter are both discussed and are unhealthy consequences of smoking. Nitrogen oxide charts. (This explanation pertains to Questions 3a to 3d in the Student Handout.) Nitrogen oxide is commonly called NOx (“knocks”), because it is mainly NO and Combustion and Emissions Teacher Notes 1 NO2. Use the charts provided to allow a class discussion (and guided note-taking) of the relationship between population, energy needs, and NOx concentrations. Highly populated areas generally have an increased demand for energy. Fuels are combusted to create most of this energy, and no combustion is perfect. Therefore, there are more nitrogen oxides released in locations with more people and more energy needs. Note the date where nitrogen oxides level off, sulfur dioxide peaks, and VOC peaks. The early 1970s were the beginning of environmental legislations in the United States. This would be a good opportunity to discuss this history of, as well as current environmental laws. Discuss ways that students and their families can reduce NOx emissions, such as carpooling, having regular car tune-ups, turning off lights that are not being used, reading books or playing sports instead of playing video games and watching television, etc. Part 2 Notes Demonstrations. Use caution with these demonstrations, since fire is involved. Set the apparatus in a safe location, and do not let students crowd around. Do not get the vacuum filter too close to the flame. Practice the demonstrations in advance. Candle Demo (This explanation pertains to Questions 4a to 4c in the Student Handout.) A fuel, oxygen (O2), and heat are needed for combustion to occur. This is known as the “fire triangle.” The candle wax is burning as the fuel. Candles are often made of petroleum, soy, or beeswax. Oxygen is present in the air around the candle. Heat is provided by the match or lighter. Combustion of the candle wax uses O2 and produces CO2. When the CO2 replaces enough O2 the flame will go out. Notes on Additional References The journal article listed below is an excellent resource for additional classroom experiments involving combustion. Birk, James P.; Lawson, Anton E. The persistence of the candle-and-cylinder misconception. Journal of Chemical Education 1999, 76(7), 914-916. The following web pages provide additional information involving topics related to combustion, specifically topics related to combustion in relation to transportation technology. EPA’s Plain English guide to the Clean Air Act. http://www.epa.gov/oar/oaqps/peg_caa/pegcaain.html EPA’s Transportation, Air Quality, and Climate Change page. http://www.epa.gov/otaq/index.htm HowStuffWorks: Global warming http://science.howstuffworks.com/global-warming2.htm HowStuffWorks: Car engines http://science.howstuffworks.com/engine.htm Popular Mechanics: Automotive technology articles http://www.popularmechanics.com/automotive/auto_technology Notes on Student Misconceptions Concerning Combustion Combustion and Emissions Teacher Notes 2 There are many common misconceptions that students bring into the classroom, and it is a good idea to stress the true science that is occurring. Below are corrections to some of these common misconceptions: Candle flames extinguish in a closed container before consuming all available oxygen, about 14-17% oxygen in air is left. Every flame requires an oxidizer, fuel, and heat (known as the “fire triangle”). The familiar shape of a flame is due to convection from air currents at different temperatures. A flame in zero-gravity is spherical!!! Water is a product of combustion and can be seen on a cold metal spoon or a cold glass held a few inches above the flame. The fuel in a burning candle is the wax. When heated, it changes from solid (the candlestick itself), to liquid (the hot wax), then to gas. The gaseous wax burns when it hits its ignition temperature in the presence of enough oxygen. Liquid wax is carried up to the hot area of the flame where it evaporates and then burns. Notes on materials Materials are common household goods. They can be found in department or hardware stores. Combustion and Emissions Teacher Notes 3 Name:___________________________ Combustion and Emissions (Chemistry of combustion, emissions from automobiles, & primary and secondary pollutants) Overview The activities and presentations in this lesson discuss combustion, its relation to energy production and its environmental effects. Use the worksheets to follow along with important topics. Part 1 (Lecture activities) 1) After discussing the presentation that was given by your teacher, answer the following questions. a) Name three examples of situations where combustion provides energy. b) If the combustion in an automobile were perfect, then these are the emissions that would occur. Balance the chemical equation. ____C8H16 + ____O2 ____CO2 + ____H2O c) The emissions from an automobile are not based on perfect combustion. Write the symbols or names for three emissions that cause pollution. d) Choose two pollutants and write about their health effects or their environmental impact. e) PM is an abbreviation for what type of emission? f) What are two major gases responsible for the greenhouse effect? Combustion and Emissions Penn State University GREATT Project Student Handout 1 Name:___________________________ Combustion and Emissions (Chemistry of combustion, emissions from automobiles, & primary and secondary pollutants) Lung Attack 1) After doing the interactive web activity about respiration, answer the following questions. a) In normal respiration, what gas goes into the bloodstream? b) In normal respiration, what gas comes out of the bloodstream? c) Fill in the table below for the types of pollution you observed: Type of Pollution Source Effect on your body d) Which of these pollutants do you think you breathe regularly? Why? Nitrogen oxides 2) After discussing the presentation concerning NOx pollution, answer the following questions. a) What is meant by the term “NOx”? b) Highly populated areas have high concentration of NOx. Why? c) What environmental effects are likely in these highly populated areas? d) What health effects are likely suffered by individuals in these populated areas? Combustion and Emissions Penn State University GREATT Project Student Handout 2 e) How do you think we can help reduce nitrogen oxide emissions? Part 2 3) After watching your teacher’s demonstrations, answer the following questions. a) What three things are needed for combustion to occur? Write these in the table below. Combustion requires…. A B C b) What happened when the burning candle was covered? c) Why does the candle burn out? Make a prediction and devise an experiment to test that prediction. Write your procedure in the box below. (You might want to look at a few additional resources before answering this question.) Proposed Procedure d) What is the earth’s major source of energy? e) How is that energy stored in nature? Combustion and Emissions Penn State University GREATT Project Student Handout 3 f) What happens when that energy is used? g) What types of products do you use that release polluting emissions and how often do you use them? h) What can you do to decrease the amount of emissions for which you are personally responsible. Part3 4) Now reflect on what you did during these activities. What do you feel that you have learned? Is there something that you still do not understand? Write your thoughts in one or two paragraphs. Combustion and Emissions Penn State University GREATT Project Student Handout 4 Part 4 5) Combustion and emissions are important scientific topics. We burn fossil fuels each day to power our cars and produce electricity for our homes and businesses. Research a topic of combustion and emissions that interests you and prepare a report to give to your class. Here are some suggestions for getting started: a) Google-search (www.google.com) some of the keywords provided in the Additional Resources section of this handout b) Check at your school or public library to see if any of the books provided in Additional Resources are available. c) Follow the URL links provided in Additional Resources that reference specific topics. Combustion and Emissions Penn State University GREATT Project Student Handout 5 Additional Resources The following keywords may help you learn more at your school or local library: Combustion Catalytic converter Air quality Fuel cell Clean Air Act Alternative fuels Air pollution Emissions Greenhouse effect Gas mileage Hybrid automobile Public transportation These books are also good resources: The Cartoon Guide to the Environment by Larry Gonick and Alice Outwater, published by Harper Resource. 50 Simple Things Kids Can Do to Save the Earth, 1992, John Javna and the Earthworks Group, Andrews and McMeel, Kansas City. The Next Step: 50 More Things You Can Do to Save the Earth, 1991, The Earth Works Group, Earthworks Press. The Kid's Guide to Social Action, 1991, Barbara A. Lewis, Free Spirit Publishing, Inc., Minneapolis. Easy Ways To Save Gas & Save Money: How To Fight High Gas Prices by Mel Leiding, 2001, Avenir International Publishing The Carbon War: Global Warming and the End of the Oil Era by Jeremy K. Leggett, 2001, Routledge Publishing On the web: EPA’s Plain English guide to the Clean Air Act. http://www.epa.gov/oar/oaqps/peg_caa/pegcaain.html EPA’s Transportation, Air Quality, and Climate Change page. http://www.epa.gov/otaq/index.htm HowStuffWorks: Global warming http://science.howstuffworks.com/global-warming2.htm HowStuffWorks: Car engines http://science.howstuffworks.com/engine.htm Popular Mechanics: Automotive technology articles http://www.popularmechanics.com/automotive/auto_technology Combustion and Emissions Penn State University GREATT Project Student Handout 6