Aerosols
advertisement
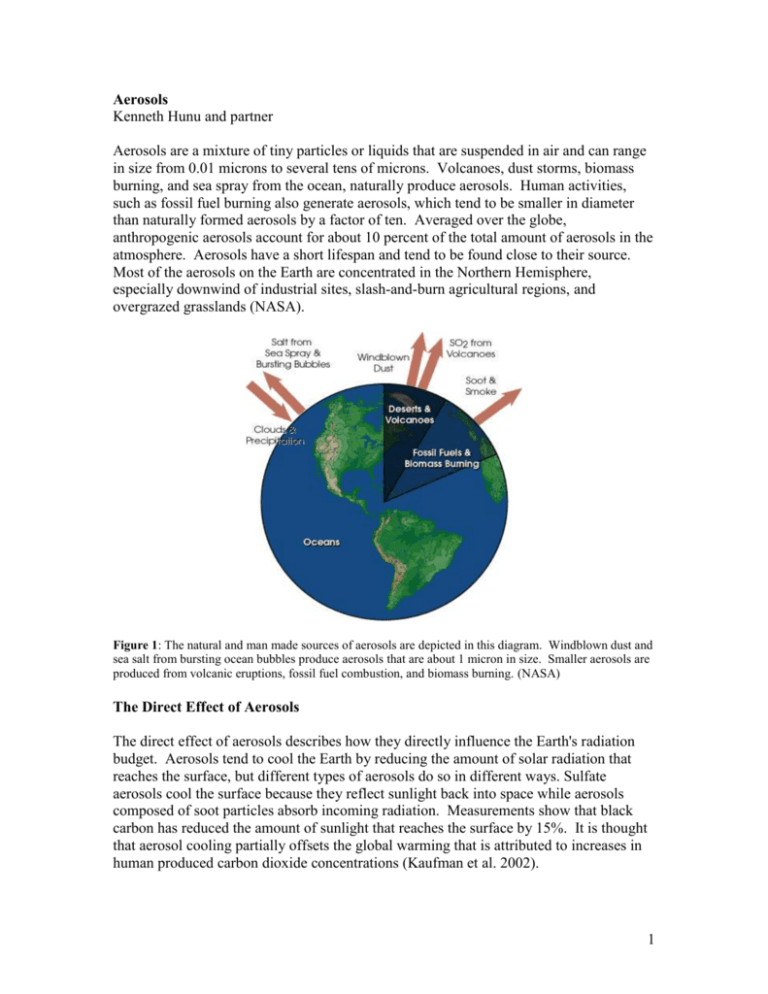
Aerosols Kenneth Hunu and partner Aerosols are a mixture of tiny particles or liquids that are suspended in air and can range in size from 0.01 microns to several tens of microns. Volcanoes, dust storms, biomass burning, and sea spray from the ocean, naturally produce aerosols. Human activities, such as fossil fuel burning also generate aerosols, which tend to be smaller in diameter than naturally formed aerosols by a factor of ten. Averaged over the globe, anthropogenic aerosols account for about 10 percent of the total amount of aerosols in the atmosphere. Aerosols have a short lifespan and tend to be found close to their source. Most of the aerosols on the Earth are concentrated in the Northern Hemisphere, especially downwind of industrial sites, slash-and-burn agricultural regions, and overgrazed grasslands (NASA). Figure 1: The natural and man made sources of aerosols are depicted in this diagram. Windblown dust and sea salt from bursting ocean bubbles produce aerosols that are about 1 micron in size. Smaller aerosols are produced from volcanic eruptions, fossil fuel combustion, and biomass burning. (NASA) The Direct Effect of Aerosols The direct effect of aerosols describes how they directly influence the Earth's radiation budget. Aerosols tend to cool the Earth by reducing the amount of solar radiation that reaches the surface, but different types of aerosols do so in different ways. Sulfate aerosols cool the surface because they reflect sunlight back into space while aerosols composed of soot particles absorb incoming radiation. Measurements show that black carbon has reduced the amount of sunlight that reaches the surface by 15%. It is thought that aerosol cooling partially offsets the global warming that is attributed to increases in human produced carbon dioxide concentrations (Kaufman et al. 2002). 1 The First Indirect Effect By changing the properties of clouds, aerosols indirectly change the albedo of the Earth. Clouds need cloud condensation nuclei (CCN), which mostly consist of aerosol particles in order to form because cloud droplets need a surface onto which they can coalesce. Increases in aerosol concentration from man-made sources produce clouds with more, but smaller droplets because if the water vapor is held constant, the water vapor is spread across a larger surface area. Droplet size has seen to be reduced by 20-30% in polluted regions. This results in an increase in a cloud's optical thickness, at about the cubed root of the difference in cloud droplet number. This increases the Earth's albedo and reduces the amount of radiation that reaches the surface, thereby cooling it (Andreae et al. 2005, Kaufman et al. 2002, Penner et al. 2006). The Second Indirect Effect As seen in the first indirect effect, increases in aerosol concentration lead to more, but smaller droplets. Smaller cloud droplets tend not to fall out as raindrops, resulting in lowered precipitation efficiency. This results in an increase in the lifespan of a cloud and increases the amount of time that the cloud can reflect sunlight. Quantifying the second indirect effect is difficult because of the already existing complexity and uncertainty of cloud dynamics and processes (Penner et al. 2006). Figure 2: The image on the left shows a cloud with low aerosol concentration. This results in the formation of a few large droplets and allows a significant portion of solar radiation to pass through the cloud. The image on the right shows a cloud with a higher concentration of aerosols in it. This results in the formation of many small droplets. This increases the albedo of the cloud and the cloud reflects more sunlight than the cloud on the left.(NASA) The Semi-Direct Effect Aerosols, such as black carbon and soot that absorb sunlight will warm the atmosphere. This reduces the sunlight that reaches the surface, but also increases the temperature of the mid-to-upper troposphere. This causes the lapse rate to decrease and stabilizes the boundary layer. This reduces the convection that occurs in the atmosphere and hinders cloud formation. In addition, soot particles that exist within a cloud can warm the surrounding area and lead to the evaporation of a part of the cloud. This reduces the 2 lifespan of the cloud and causes a warming effect on the planet because the amount of cloud cover on the planet is reduced (Andreae et al. 2005, Ackerman et al. 2000). Aerosols and Ozone depletion Aerosols may also act as sites for chemical reactions to take place (heterogeneous chemistry). During winter in the stratosphere in the Polar Regions, aerosols grow to form polar stratospheric clouds. The large surface areas of these cloud particles provide sites for chemical reactions to take place. These reactions produce large amounts of reactive chlorine that lead to the destruction of stratospheric ozone (NASA). Figure 3: After a volcanic eruption, large amounts of sulfur dioxide (SO 2), hydrochloric acid (HCl) and ash are spewed into the Earth's stratosphere. Hydrochloric acid, in most cases, condenses with water vapor and is rained out of the volcanic cloud formation. Sulfur dioxide from the cloud is transformed into sulfuric acid (H2SO4). The sulfuric acid quickly condenses, producing aerosol particles which linger in the atmosphere for long periods of time. The interaction of chemicals on the surface of aerosols, known as heterogeneous chemistry and the tendency of aerosols to increase levels of chlorine, are prime contributors to stratospheric ozone destruction. (NASA) Evidence for anthropogenis aerosols and the effects of aerosols on climate forcing: 1. Sulfur dioxide forms sulfate aerosol particles in the atmosphere, which causes the clouds to be more reflective, carry more water and possibly stop precipitation. This has been ascertained by the study of “ship tracks”. This is proof that humans have been creating and modifying clouds for generations through the burning of fossil fuels and explains why global warming is affecting the Southern Hemisphere much more quickly than the Northern Hemisphere (King et al. 1993). 2. Regional variability of fine aerosols: due to the relatively short lifetime of aerosols, they tend to remain at their source areas. Fine hygroscopic particles (urban haze) are found downwind of populated regions in polluted (from industrial and burning of fossil 3 fuels) areas like China. Black carbon is also concentrated at regions where much burning takes place. These indicate that they are anthropogenic. Figure 4: (a) shows the global distribution of fine aerosols mainly as a result of pollution and vegetation fires whereas figure (b) shows the global distribution of course aerosols which are mainly dust and salt.(EESC W4400 lecture notes) 3. Cloud base: aircraft measurements show that in polluted air a six-fold increase in the number of fine aerosols per unit volume of air produces a three-to fivefold increase in the droplet concentration. Analysis of global satellite data shows that such a change in aerosol concentration corresponds to 10-25% smaller cloud droplets because the condensed water is divided into more numerous droplets. These clouds therefore have a larger surface area and hence a larger reflectivity. What do model simulations of past/present indicate? Models show that the aerosol forcing ranges from 0 to - 4.4 W/m2(IPCC). Furthermore, they seem to have presently offset greenhouse gases by 25-50% (Kaufman et al. 2002). What are the models suggesting for the future, and what are the assumptions in those projections? Using an aerosol forcing of -1.5 W/m2, models using several IPCC scenarios show a difference in climate sensitivity of up to 6ºC for 2100 between a strong aerosol forcing situation and one with no aerosol cooling effect (Andreae et al. 2005). One potential weakness of the GCMs used is that their resolution may be too low to depict the dynamics and processes of clouds well (Penner et al. 2006). Differences also occur because modelers have to assume how aerosols interact with water vapor and how water vapor will be distributed in the atmosphere. Since cloud processes are not known well, the used assumptions affect the uncertainty of the calculated impact aerosols have on climate. Each model also decides what cloud droplet number concentration to use and how to predict future aerosol concentration changes (Penner et al. 2006). Another model, which also takes into account future decreases in sulfate aerosol concentrations but uses 4 one IPCC scenario (SRES-A2), shows differences in temperature increases of 3.5ºC within the 21st century when both man-made reflective aerosol concentration changes are included and when they are not. This insinuates that sulfate aerosols may not play a large role in cooling the Earth in the 21st century, especially considering the large greenhouse gas increase that is predicted to occur. Their models also show that during the first half of the 21st century, greenhouse gas forcing increases much faster than in the 20th century while aerosol forcing remains constant or becomes more positive. Forcing of sulfate aerosols from 1945-1995 is about -.05, while it becomes almost zero from 1995-2045. Their models also show that the aerosol forcing becomes positive (0.5) as the emissions of sulfate decrease during 2045-2095 (Dufresne et al. 2005). What are the principal uncertainties in the scientific evidence and in the forecasts? Scientists disagree about the magnitude of aerosol forcing, but they all agree that aerosols have a net cooling effect. This large uncertainty, which ranges from 0 to -4.4 W/m2, is due to several factors. Scientists are still unclear about how clouds respond to aerosol concentrations. In addition, they cannot exactly determine how clouds respond to concentrations of aerosols. A substantial amount of information about cloud dynamics and behavior is still unknown. This also makes it difficult to depict cloud processes in models that predict future aerosol forcing and future temperature changes of the Earth (Andreae et al. 2005, Kaufman et al. 2002). There are currently two main methods that are used to calculate aerosol forcing, forward and inverse. Forward calculations are based on physical and chemical properties of aerosols and use that information to determine what effect aerosols have on the Earth's radiation budget. Inverse calculations work backwards from model simulation results to determine what portion of the Earth's total forcing is attributable to aerosols. Inverse calculations result in an uncertainty range less than 2 W/m2, with an average forcing of -1 W/m2. Forward calculations have an aerosol forcing that ranges from 0 to about -5 W/m2. There is no clear explanation as to why such a disparity exists, as fairly well defined physics and observations are used in both types of calculations (Anderson et al. 2003). Thus, more reliable observations about aerosol emission rates, their lifetimes, and their concentrations are needed to improve these predictions. In addition, there is a need to improve the understanding scientists have about the impact aerosols have on the hydrologic cycle, specifically how they affect clouds (Ramanathan et al. 2001). In addition, this uncertainty about the net radiative forcing of aerosols makes it difficult to estimate the Earth's climate sensitivity. In order to better determine how the Earth will respond to increases in greenhouse gases, especially if aerosol concentrations change dramatically in the future, it is important to understand how strong of an effect aerosols have on the Earth's radiation budget. 5 Figure 5: This figure from the IPCC shows how low the understanding about aerosols is and how the forcings from the first indirect effect and mineral dust are difficult to quantify (IPCC). Main Points Aerosols affect the Earth’s radiation budget and currently have a net cooling effect by directly or indirectly changing the amount of solar radiation that reaches the Earth’s surface. In the future, aerosol cooling is expected to decline relative to greenhouse gas forcing. High concentrations of small aerosols, mostly anthropogenic in source, change cloud properties and thus, affect the hydrologic cycle by reducing the precipitation efficiency of clouds. It is uncertain how large the aerosol forcing is even though aerosols have an estimated radiative forcing of about 0 to -4.4W/m2. This is partly due to our incomplete understanding of cloud dynamics. References Ackerman, A.S., Toon, O.B., Stevens, D.E., Heymsfield, A.J., Ramamathan, V., Welton, E.J. (2000). “Reduction of Tropical Cloudiness by Soot.” Science, 288, 1042-1047. Anderson, T.L., Charlson, R.J., Schwartz, S.E., Knutti, R., Boucher, O., Rodhe, H., Heintzenberg, J. (2003). “Climate Forcing by Aerosols—a Hazy Picture.” Science, 300, 1103-1104. 6 Andreae, M.O., Jones, C.D., Cox, P.M. (2005). “Strong present-day aerosol cooling implies a hot future.” Nature, 435, 1187-1190. Boer G.J. et al(2000) “A transient climate change simulation with greenhouse gas and aerosol forcing: projected climate change to the twenty-first century” climate dynamics,427-450. Published by Springer Berlin/Heidelberg. Dufresne, J.L., Quaas, J., Boucher, O., Denvil, S., Fairhead, L. (2005). “Contrasts in the effects on climate on anthropogenic sulfate aerosols between the 20th and 21st century.” American Geophysical Union, 32, L21703. Intergovernmental Panel on Climate Change (2006). “Anthropogenic and natural forcing of the climate for the year 2000, relative to 1750.” <http://www.ipcc.ch/present/graphics/2001syr/small/06.01.jpg> Kaufman, Y.J., Tanre, D., Boucher, O. (2002). “A satellite view of aerosols in the climate system.” Nature, 419, 215-222. National Aeronautics and Space Administration (2006). “EO Library: What are Aerosols? Fact Sheet.” <http://earthobservatory.nasa.gov/Library/Aerosols> NASA facts online “Atmospheric aerosol, what are they and why are they so important?” <http://oea.larc.nasa.gov/PAIS/Aerosols.html> Radke's et al “Direct and Remote Sensing Observations of the Effects of Ships on Clouds” Science, Vol 246, 1989 Ramanathan, V., Crutzen, P.J., Kiehl, J.T., Rosenfeld, D. (2001). “Aerosols, Climate, and the Hydrological Cycle.” Science, 294(7): 2119-2124. Penner J.E, Chuang C.C, Grant K.(1998). “Climate forcing by carbonaceous and sulfate aerosols” Climate Dynamics, 839-851. Published by Springer Berlin/Heidelberg. Penner, J.E., Quaas, J., Storelvmo, T., Takemura, T., Boucher, O., Guo, H., Kirkevag, A., Kristjansson, J.E., Seland, O. (2006). “Model intercomparison of indirect aerosol effects.” Atmospheric Chemistry and Physics, 6, 3391-3405. 7







