Exercise #1 PHYC 251: General Physics
advertisement

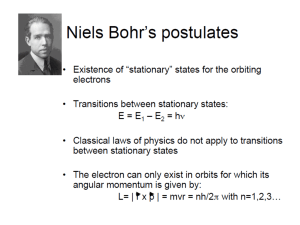
Physics 110 Physical Science Name _______________ Date ________________ Exercise 12: Atomic Physics Check dimensions and proper significant figures. Planck’s constant h = 6.626x10−34 J–s e = 1.6x10−19 coulombs (C) Quantized Charge 1. An object has a net charge q of −3.2x10–13 coulombs. How many more electrons are there than protons (q = Ne)? Quantized Light 2. A green laser pointer emits photons of frequency 5.64x1014 Hz. What is the energy of one photon (Eonephoton) associated with the laser? Hydrogen atom Bohr radius r1 = 0.053 nanometers (nm) Hydrogen ground state E1 = −13.60 electron volts (eV) 3. What is the radius of the electron orbit for a hydrogen atom for n = 3? The various Bohr energy levels for the electrons in the hydrogen atom are E1 = −13.6 eV E2 = E1/4 = −3.40 eV E3 = E1/9 = −1.51 eV If an electron jumps from the n = 3 to n = 2 level, a. Is it absorbing or emitting a photon? b. What is the change in energy (in eV)? c. Use the conversion factor 1 eV = 1.6x10−19 J to calculate the energy change in joules. d. Using Planck’s constant h, calculate the frequency of the photon. a) 4.6x1014 Hz b) 6.2x1013 Hz c 7.3x1013 Hz d. None of the above. 1