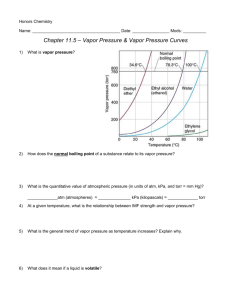
The graph shown below represents a sample heating curve for a
advertisement

HW - HEAT CURVE NAME_______________________ The graph shown below represents a sample heating curve for a substance. Use it to answer the questions below: 90 E 80 T E M P (oC) 70 D 60 50 40 C 30 20 10 B 0 -10 A 10 20 30 40 50 60 70 80 90 100 110 120 TIME (min) ________________________ 1) What phase (or phases) are present during segment A? ________________________ 2) segment B? ________________________ 3) segment C? ________________________ 4) segment D? ________________________ 5) segment E? ________________________ 6) What phase change, if any is occuring during segment A? ________________________ 7) segment B? ________________________ 8) segment C? ________________________ 9) segment D? ________________________ 10) segement E? ________________________ 11) Which H equation would you use to calculate the heat absorbed by the substance during segment A? ________________________ 12) segment B? ________________________ 13) segment C? ________________________ 14) segment D? _______________________ 15) segment E? _______________________ 16) What is the melting point for this substance? _______________________ 17) What is the boiling point for this substance? _______________________ 18) How long did it take for the substance to heat up to the melting point? _______________________ 19) How long did it take for the substance to melt? _______________________ 20) How long did it take for the substance to heat up to the boiling point? _______________________ 21) How long did it take for the substance to boil? HW - HEAT CURVE NAME_______________________ 22) Vapor Pressure and Boiling. The following graph shows vapor pressure curves for two substances, A and B: Vapor Pressure Curve for Two Substances 160 150 140 130 120 110 Vapor pressure (kPa) 100 A 90 80 70 B 60 50 40 30 20 10 10 50 100 Temperature (oC) ___________ a) What is the vapor pressure (in kPa) of A at 30 oC? _____________ b) What is the vapor pressure of B at 30oC? _____________ c) At what temperature is the vapor pressure of A 80 kPa? _____________ d) What is the vapor pressure of B at the temperature from (c)? _____________ e) At what temperature is the vapor pressure of B equal to 80 kPa? _____________ f) Boiling of a liquid occurs when the vapor pressure equals what quantity? _____________ g) The “normal boiling point” is the boiling temperature at standard pressure. What is standard pressure in kPa? _____________ h) What is the normal boiling point of A? _____________ i) What is the normal boiling point of B? _____________ j) At what temperature would A boil in Denver, CO if the atmospheric pressure there were 90 kPa? _____________ k) What would the atmospheric pressure have to be for B to boil at the temperature in (j)?