Experiment 2-4
advertisement

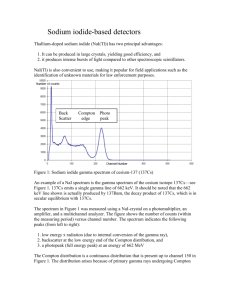
PHYSC 3622 Experiment 2.4 6 February, 2016 Gamma ray spectroscopy and absorption Purpose In this experiment, you will investigate the spectra of gamma rays emitted from radioactive nuclei and the attenuation of gamma rays in lead. Part I Gamma ray spectroscopy Background Radioactive nuclei decay by a number of modes, such as alpha or beta emission. These processes frequently involve the emission of energetic photons as well; we call these photons gamma rays. Their energies range from tens of keV up to a few MeV. One example is the decay of Cs 137 , shown below: 55 Cs 137 56 Ba 137 (1) Approximately 94% of the time, the beta particle (electron) is emitted with 511 keV of energy and the Ba nucleus is left in an excited state. The Ba nucleus then goes to the ground state by quickly giving off a 662 keV gamma ray or photon. We are interested in this photon. (In the remaining 6% of transitions, the nucleus goes directly to the ground state and the electron is emitted with 1.17 MeV of energy.) If we were to measure the intensity and energy of gamma rays emitted by a Cs 137 source, we would expect to observe a single peak at 662 keV. As we shall see below, the real situation is somewhat more complicated. Our problem is to measure the intensity of photons (gamma rays) as a function of energy or wavelength. We will use a scintillation counter pulse-height technique that directly measures the intensity versus energy. Figure 1 shows a block diagram of the apparatus. Figure 1. Block diagram of the gamma ray spectroscopy system. The gamma ray enters the Tl-doped NaI crystal detector where it is absorbed in accordance with the photoelectric effect and converted into a pulse of light. The peak intensity of the pulse is proportional to the energy of the gamma ray. This light pulse is then converted into a current pulse by the photomultiplier tube (PMT). Again, the 1 PHYSC 3622 Experiment 2.4 6 February, 2016 height of the current pulse is proportional to the energy of the gamma ray. The current pulse is amplified and the resulting voltage signal is fed to a "sample-and-hold" circuit, which generates a steady output voltage proportional to the peak input voltage. Next, this voltage is converted to a number between 0 and 255 by an analog-to-digital converter (ADC). The number generated by the ADC is once again proportional to the voltage. Finally, this number forms the address of a single channel in the multi-channel analyzer (MCA). The MCA has 256 channels, one for each address. Each channel is essentially a digital counter, and the count in the selected channel is incremented by one. Each channel in the MCA can hold up to 16,777,215 ( 2 24 1 ) counts. Using this technique, we are able to generate a plot of the number of counts (proportional to the gamma ray intensity) versus channel number (proportional to gamma ray energy). By running several different sources having known emission energies, we can make a calibration curve giving energy as a function of channel number. The MCA contains circuits that drive a video display and that allow us to send the data to a computer, so that we can make a hard copy. A booklet published by The Nucleus, Inc., entitled Spectrometry Experiments with Multichannel Analyzers gives a more complete description of the operation of the MCA. Note that our Nucleus 800 MCA is a bit different from the one described in the booklet (for instance, the high voltage power supply for the PMT is fixed at 1000 V). From the above discussion, we might expect that a plot of gamma ray intensity versus energy would show a single peak at the proper energy (662 keV for Cs 137 ). Instead, the situation is more complex, as shown by the sample spectrum in Figure 2. The photon is subject to Compton scattering (an elastic process) by one or more of the electrons in the material. Thus, the photon may lose energy before it is absorbed photoelectrically. Compton scattering is derived in the booklet and in any modern physics text; the energy h of the scattered photon is given by h h 1 h / m0 c 2 1 cos , (2) where h is the energy of the incident photon, is the scattering angle of the outgoing photon, and m 0 c 2 is the rest energy of the electron. The kinetic energy Te of the scattered electron is just Te h h . (3) The electron receives maximum kinetic energy when the photon scattering angle is 180°. Compton scattering gives rise to a smoothly distributed spectrum out to the Compton edge and, frequently, a back scattering peak, as shown in Figure 2. Other processes, which complicate the spectrum, also take place; some of them are discussed in the booklet. Once they are emitted, gamma rays and x-rays are the same thing. A gamma is a photon emitted from the nucleus, while an x-ray comes from the electron cloud surrounding the nucleus. However, the detector cannot distinguish between them. In fact, the type of system you are using in this experiment is often attached to a scanning electron microscope to measure the energies of the x-rays induced by the electron beam. A computer then analyzes the spectrum and gives a printout of the chemical elements present in the sample. (The American Institute of Physics Handbook, Chapter 8, is a good reference.) 2 PHYSC 3622 Experiment 2.4 6 February, 2016 Figure 2. The upper graph shows counts versus channel number for a Cs 137 source, while the lower graph shows the same data plotted as counts versus energy. 3 PHYSC 3622 Procedure Experiment 2.4 6 February, 2016 Energy calibration: To begin, place the Cs 137 source in the middle tray beneath the detector. Run several curves using different gain settings to get a feel for the instrument. Next, do your calibration runs. Gain settings of Coarse = 20 and Fine = 1.1–1.3 are suggested. Run up 1000–2000 counts on the principal peaks for each of the following sources: Cs 137 (662 keV), Co 60 (1.173 & 1.332 MeV) and Ba 133 (356 & 302 keV). Use the marker to indicate the peak on the screen and record the channel number corresponding to the peak in your notebook. Using Sigma Plot, perform a linear regression analysis to relate the energy to the channel number, obtaining an equation of the form E mN b , (4) where E is the peak energy and N is the channel number; m (slope) and b (intercept) are fitting parameters. From your practice runs, you should have noticed that the peak channel number is a function of the gain settings, so be sure not to change the gain settings once you have begun to take "real" data. Printing a spectrum: To print a spectrum, start by running the Gamma Ray Spectroscopy program on the PC. To send data to the computer, press the Input/Output Mode button on the MCA control panel, so that the video display reads SERIAL OUTPUT at the top. Then press the Start button at the computer and the Start/Stop button at the MCA to send the data to the computer. After a few seconds, the computer display should show the spectrum. At the computer, you can change the horizontal axis from Channel Number to Energy and back by pressing the vs. Energy/vs. Channel button. However, you won't be able to do this until you enter the calibration constants you obtained above. Press the Calibrate button to enter the data. You can get a hard copy of the spectrum displayed on screen by pressing the Print button. Questions Run the "unknown" source. Read the data into the computer and make a plot of counts versus energy. Use your data and a table of the radionuclides to identify the nuclei present in the source. Hint: The unknown source is a mixture of two isotopes, one of which you've already looked at, along with a second isotope you haven't yet seen. Run Cd 109 . Plot the data. What do the data show? What is the resolution of the instrument? Note that each channel gives the number of events having energy between E and E dE , where dE is the resolution. Now recalibrate the instrument for a lower maximum energy. Suggested gain settings are Coarse = 20 and Fine = 2.0–2.4. Use the Cs 137 and Ba 133 sources to calibrate the energy scale. Repeat the Cd 109 spectrum and make the appropriate plots (don't forget to enter the new calibration values into the computer). What do they show? What is the resolution of the instrument now? Part II Gamma ray absorption Background In most materials, gamma rays are attenuated exponentially, so that their intensity can be expressed as 4 PHYSC 3622 Experiment 2.4 6 February, 2016 I I 0 e x , (5) where I 0 is the incident intensity, is the absorption coefficient, and x is the distance. Usually, is reported in units of cm 1 and x in units of cm . We often use the mass absorption coefficient m , which is defined as m / , (6) where is the density of the material. The goal of this experiment is to measure m as a function of energy for lead. Procedure Figure 3 shows the arrangement of the source, the lead absorber(s) and the detector. Set up the multichannel analyzer with Coarse set at 20 and Fine at 1.1–1.3. These settings usually give a good readout of the Cs 137 spectrum. Select a running time that gives about 2000 counts with the Cs 137 source placed in the middle tray. Use the on-screen cursor to read the number of counts at the 0.662 MeV peak of the spectrum, and record the time duration of the run as well. Now repeat the runs using the Pb absorbers, using enough different combinations of thicknesses to obtain several points in the range of 0– 1.5 cm. The durations of these runs should be about the same as for the first (no absorber) run. Divide the peak counts by the duration for each run, in order to normalize the data and give intensity. Plot your data as the logarithm of intensity vs. Pb thickness, using Sigma Plot. Determine the slope of a least-squares straight line fit to the data, and calculate the resulting value of at the Cs 137 energy of 0.662 MeV. Repeat the runs for Co 60 (both peaks) and Na 22 to obtain the values of at other energies. Calculate the value of m for each , and plot m vs. energy. Compare your results with handbook values. Figure 3. Arrangement of source, absorber and detector. Questions Describe how your results differ from the handbook values, if at all. Discuss the discrepancies and suggest possible sources of error. 5