MRI Notes - Charles Sturt University
advertisement

SCHOOL OF CLINICAL SCIENCES
MIS320 RADIOLOGICAL INSTRUMENTATION 4
PHY336 MAGNETIC RESONANCE IMAGING
MRI PHYSICS & INSTRUMENTATION
NOTES
Radiological Instrumentation 4
Magnetic Resonance Imaging
MIS320
PHY336
Radiological Instrumentation 4
Magnetic Resonance Imaging
Faculty of Health Studies
Authors
Hans Swan
Rob Davidson
i
Instructional design
Elizabeth Miller
Text processing
Jayne Taylor
Produced by Open Learning Institute, Charles Sturt University, Albury - Bathurst - Wagga-Wagga,
New South Wales, Australia.
Published
Revised
December 1997
December 1998
Printed at Charles Sturt University
Charles Sturt University
Previously published material in this book is copied on behalf of Charles Sturt University pursuant
to Section 40(1A) of the Commonwealth Copyright Act 1968 as amended.
ii
Contents
Page
1
2
Introduction
1
1.1
1.2
1.3
1
2
3
The basic physics of MRI
2.1
2.2
2.3
2.4
2.5
2.6
2.7
2.8
2.9
2.10
2.11
2.12
3
Simplified overview of the MRI process
Nuclear magnetic resonance (NMR)
MRI pros and cons
The atomic nucleus in a magnetic field
The radiofrequency (RF) pulse : Its purpose and application
The magnetisation vector & its manipulation in MRI
The production of the 90 pulse
Emission of NMR signal & free induction decay (FID)
Transverse relaxation (or “Spin-spin” or “T2” relaxation)
T2 Relaxation (pronounced “ tee-two star” )
Longitudinal Relaxation (or “Spin-lattice” or “T1” relaxation)
Spin density
Measurement of T2, & T2-weighted images
Measurement of T1, & T1-weighted images
TR and TE choices for T2- and spin-density-weighted images
4
4
12
15
20
21
23
24
26
28
28
34
39
Echo sequences and image generation
42
3.1
3.2
3.2
3.3
3.4
42
43
44
45
52
The spin echo sequence
Multiple echo sequences
Multiple slice imaging
Spatial localisation
Scan time
4
Self-assessment questions
58
5
Recommended references
61
iii
iv
MRI Physics & Instrumentation Notes
1
Introduction
This module seeks to introduce you to the basic principles governing the medical
imaging modality known as Magnetic Resonance Imaging (MRI). Although very
different in purpose to Nuclear Medicine imaging, MRI also depends on the
behaviour of atomic nuclei, and employs the emission of radiation from within the
body. Unlike Nuclear Medicine, MRI is more akin to CT scanning which
produces tomographical images depicting organ structure rather than function.
MRI is one of the most exciting and important developments in medical diagnosis
since the discovery of X - rays over 100 years ago. It produces some of the best
contrast resolution possible in images of human soft-tissue structures. It is very
different in its operating principles to X-radiography and Ultrasound, and is based
on the response of certain atomic nuclei situated in a magnetic field to
electromagnetic energy at a radio frequency.
The diagnostic scope of MRI is much greater than X-ray CT in soft-tissue
imaging. Nevertheless it is complementary to CT and all the other medical
imaging modalities. In some applications it is the most suitable, if not the only
method for proper diagnosis. Areas of significant application lie in brain and
spinal imaging.
1.1 Simplified overview of the MRI process
The following sequence of events very simply describes the chief processes
involved in the production of an MR image:
1.
The patient is placed in a magnetic field.
2.
A radio frequency (RF) electromagnetic wave (of pre-determined frequency)
is produced and radiated into the patient. (The frequency range utilised in
MRI lies between 1 MHz and 80 MHz).
3.
The applied radiowave is turned off.
4.
The patient re-radiates the absorbed RF signal, which is then detected by a
receiver.
5.
The received signal is used to reconstruct an image with the aid of a
computer.
1
MRI Physics & Instrumentation Notes
These essential processes are illustrated in Figure 3.1 below:
RF signal
Transmitter
Organ
in
Magnetic
Field
Magnet
emitted RF signal
Computer
Image
Receiver
Figure 3.1
(Adapted from Newhouse & Wiener)
1.2 Nuclear magnetic resonance (NMR)
MRI is possible because of a phenomenon called Nuclear Magnetic Resonance
(NMR).
To understand this phenomenon, it must be understood that the nucleus of an atom
has magnetic properties (i.e. a magnetic field) associated with its “spin” and
charge distribution.
When such a nucleus is placed in a strong external magnetic field, it is possible
under the correct conditions, for it to absorb and at a later time, release a quantity
of energy unique to the nucleus and its surrounding environment: this is called
nuclear magnetic “resonance”.
Since the 1940’s, NMR has been used extensively as a spectroscopic tool in chemistry
and biochemistry research to study the content and structure of materials.
In 1972, P. Lauterbur modified the NMR spectroscope to provide spatially
encoded signals, thus allowing the first two-dimensional NMR image to be
obtained. This led to the birth of nuclear magnetic resonance imaging for
diagnostic purposes. Following the nuclear reactor disasters at Three Mile Island
and Chernobyl, the term “nuclear” was expediently dropped from name, and the
title, Magnetic Resonance Imaging (MRI) was coined.
2
MRI Physics & Instrumentation Notes
1.3 MRI pros and cons
Since the mid 1980s, MRI has gained preference over CT in many situations. The
single biggest advantage of MRI is its high contrast sensitivity to soft-tissue
differences. Its value is unparalleled in neurological examinations and is
increasingly more valued in abdominal and musculo-skeletal examinations. It also
provides inherent patient safety through its employment of non-ionising radiation,
although other safety issues arise.
The growth of MRI in the 1990s has not only been due to its excellent soft-tissue
contrast sensitivity. Uses for MR, other than anatomical imaging, have also
rapidly developed. Its improved sensitivity to flow motion (a problem in the
1980s) now allows the imaging of blood-and CSF- flow, as well as the
observation of other physiological functions. MR spectroscopy, considered as a
laboratory tool in the 1980s, is now being used in vivo. Functional mapping of
brain activity is being carried out using techniques such as perfusion and diffusion
imaging. These techniques have resulted from the development of ultra-fast
imaging, called echo-planar imaging. (The coverage of these latest developments
is beyond the scope of this subject. However, once a basic understanding of MRI
has been achieved from this subject you will be in a better position to delve into
these new developments, should you require to.)
As with any other imaging modality, MRI has its drawbacks. Equipment and
siting costs are very high; many patients must be excluded from the strong
magnetic field due to safety reasons and patient claustrophobia is not uncommon.
3
MRI Physics & Instrumentation Notes
2
The basic physics of MRI
In order to understand how to interpret and obtain the maximum information from
MRI scans, as well as to potentially obtain optimum image quality in such scans, it
is essential to grasp the fundamental physical principles underpinning this
technology. The application of some of the early concepts to the imaging process
may not be immediately apparent. Further, the physics can appear complex in
places. Nevertheless, it is not beyond comprehensionso just hang in there,
while we piece together how the principles build onto each other to produce the
MR image.
2.1 The atomic nucleus in a magnetic field
Let us first look at the atomic nucleus, and its properties and behaviour, especially
after it is put in an external magnetic field (...this eventually relates to what
happens to a patient when put into the magnetic field of an MR machine).
Because the human body is about 60 - 70% H2O, hydrogen (H) nuclei (i.e. single
protons) are the most abundant constituent of soft-tissue. All MR imaging of softtissue is based on the behaviour of the H-nucleus (protons) in magnetic fields.
Note that fats and lipids within the body also contain hydrogen atoms, and so are
imaged as well. It is possible to do MR imaging with other nuclei, but it is more
difficult than with protons.
Protons have a positive charge (+ 1.6 10-19 C). Their motion can be thought to
be analogous to little planets, in that they can be imagined to spin continuously on
their axes. We say that protons possess a “spin”. The Hydrogen nucleus can
therefore be thought of as a spinning electric charge. Such a spinning electric
charge can be thought of as a tiny circular electric current loop, “I”, as illustrated
in Figure 3.2.
spin axis
+
“I”
Figure 3.2
You will recall from elementary physics, that any electric current loop will have
associated with it an induced magnetic field, B directed along the loop axis in a
direction given by the right hand rule, as illustrated in Figure 3.3. The strength of
the magnetic field will be directly proportional to the loop current.
4
H
MRI Physics & Instrumentation Notes
B
H
B
Figure 3.3
The spinning H-nucleus will therefore effectively have a North and South
magnetic pole along its spin axis, and can be likened to a bar magnet, as shown in
Figure 3.4. The arrow in the figure is called the magnetic dipole moment
vector, µThis vector provides a measure of the orientation and magnitude of the
proton magnetic field strength.
µ
µ
“N”
N
+
S
“S”
Figure 3.4
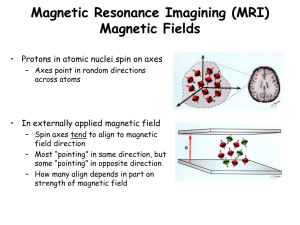
In the absence of an externally applied magnetic field, the magnetic dipole
momentsof the millions of spinning H-nuclei within the human body will be
randomly oriented, as indicated in Figure 3.5.
Figure 3.5
When body tissue is placed in an externally applied B-field, the proton dipoles
will “align” with the field (just as compass-needles will align with the Earth’s
magnetic field). However, there is one important difference between nuclear
magnetic dipoles and compass-needles, in the way they align with the applied
field. Compass-needles will align in only one possible direction, i.e. exactly along
Bearth as indicated in Figure 3.6.
5
MRI Physics & Instrumentation Notes
B
N
N
N
N
Figure 3.6
Proton dipoles, however, can align in two possible directions with respect to the
applied field. This is a result of quantum mechanics which describes the
behaviour of the microscopic world. The two possible alignments (orientations)
are shown in Figure 3.7.
B
Figure 3.7
Quantum Mechanics dictates that the alignment of the dipoles of spinning protons
cannot be exactly along the applied magnetic field direction (unlike macroscopic
dipoles such as compass-needles), but must lie at a specific angle to it. That is,
the orientations are “quantised” and no other values of are permitted.
The magnitude of the nuclear dipole moment is given in units of the “nuclear
magneton”, N:
N 5.05 10 27 J / Tesla
and the magnetic dipole moment of a proton is
p 2.7928 N
The proton dipole moment is that property of the spinning proton which makes it
behave like a microscopic bar magnet. The magnitude of the proton dipole
moment quoted above, is in fact one of the largest possessed by any nuclear
particle, which makes H-nuclei or protons one of the most appropriate for MR
imaging.
6
MRI Physics & Instrumentation Notes
The dipoles pointing “upward” and making an acute angle with B (refer to
Figure 3.7) are referred to as “spin-up”, while those in the “downward”
orientation ( obtuse) are called “spin-down” dipoles.
The two dipole orientations represent two different energy levels. Spin-up
dipoles are in the lower energy state. Spin-down dipoles are in a slightly higher
energy state. The precise occupancy rate of the two states will depend on
different factors, including the magnetic field strength, B , and temperature, but
nevertheless, is always close to 50-50. Because the lower energy (spin-up) state is
easier to attain, its population is found to be very marginally more than the higher
energy (spin-down) state. Very roughly, for about 1,000,000 protons in the spindown state, there are about 1,000,001 in the spin-up (low energy) state. Therefore,
in a sample of about 1023 Hydrogen nuclei ( ~ Avogadro’s Number - equivalent to
a fraction of a gram of H-nuclei) there would be about 1017 more spin-up than
spin-down dipoles.
It is these very “few” excess spin-up nuclei (one in a million) that are used to
produce the NMR signal from which the final soft-tissue image is constructed.
The reason only these excess dipoles may contribute to the NMR signal from a
given tissue sample, is because it is only their magnetic dipole moments which
remain uncancelled by equivalent spin-down dipoles. There is therefore a net
spin-up magnetic dipole moment which is the vector sum of the moments of all
the excess spin-up dipoles in a tissue sample. This is called the Magnetisation
Vector, M which points in the direction of the externally applied field.
To proceed to understand the behaviour of the Magnetisation Vector, M of atomic
nuclei, and how it is used in MRI, we first need to understand a little more of the
motional behaviour of atomic nuclei in magnetic fields.
We now understand that protons have an intrinsic “spin” property without which
their magnetic dipole moments could not exist. You will recall that any object of
mass m, moving at a speed v, will possess linear momentum (p), given by p mv .
For objects which rotate in a circle, linear momentum is replaced by angular
momentum, L, given by L = r mv, where r is the radius of the circle. If r and v
are as depicted in Figure 3.8, then the angular momentum vector, L will be
directed straight out of the page from the centre of the circle.
v
mass m
r
Figure 3.8
Like rotating objects, spinning objects will also possess angular momentum, with
L directed along the spin axis.
7
MRI Physics & Instrumentation Notes
In the macroscopic world, both linear and angular momentum can take on any
arbitrary value, i.e. they are continuous variables. However, in the microscopic
world of atoms and nuclei, angular momentum is quantised, according to
Quantum theory. That is, it can take on only certain allowed discrete values, and
no others. Nuclear spin angular momentum is thus described by a spin quantum
number, I (also simply called “nuclear spin”) whose magnitude depends on the
type of atom / element. The maximum detectable nuclear angular momentum for
a given element is given by:
L I ,
where =
h
( is pronounced “h-bar”, and h = Planck’s constant = 6.63 10-34 JS)
2
and where I =
1 3 5
, , ,
2 2 2
if the element has an ODD “mass no.” (i.e. odd
number of protons + neutrons).
=0…
if the mass no. and atomic no. (i.e. proton number) are
both EVEN
= 1, 2, 3, 4,… if the mass no. is EVEN but proton no. is ODD.
From the above , it can be seen that protons (H-nuclei) fall into the first category
since the mass number is odd. For protons, I = ½.
It is interesting to note that for elements with I = 0, there is no nuclear spin angular
momentum (i.e. L = 0 ). This leads to the conclusion that the nuclei of these
elements have no magnetic dipole moment. A consequence of this is that such
nuclei cannot be detected by NMR....( must exist to get an NMR signal).
We can see that the magnitude of the Angular Momentum quantum number plays
an important role in determining which elements might be useful for MR imaging,
and which will not be.
The possession of spin angular momentum by H-nuclei occupying the
“spin-up” and “spin-down” states in an external B field, has a significant effect on
the behaviour and motion of the proton dipoles. Even though the dipoles are
constrained by quantum mechanics to be oriented at a specific angle, with
respect to the applied magnetic field ( see Figure 3.7), their orientation in 3dimensional space does not remain fixed, but moves around in a certain way. This
type of motion is called Precession, as illustrated in Figure 3.9.
8
MRI Physics & Instrumentation Notes
S
B
N
Figure 3.9
This motion is similar to that of a spinning top in a gravitational field - when the
axis of spin is displaced from the vertical direction, the top starts to wobble or
tumble around the vertical axis. The spin axis circles the vertical gravitational
axis, forming a cone shape as indicated in Figure 3.10.
axis of
gravitational
field
precession
spin
top
Figure 3.10
Because spinning protons have magnetic dipole moments whose orientations are
automatically displaced by a finite angle, , with respect to the applied B field,
they will precess about the field magnetic direction.
The precessional motion of the proton dipoles will occur at a specific frequency,
depending on the strength of B and on the nature of the medium.
It is important not to confuse the frequency of proton spin (on the spin axis) with
the dipole precession frequency about the magnetic field direction. The
precession frequency is equal to the number of times the proton dipoles precess
every second around the B - direction.
9
MRI Physics & Instrumentation Notes
The proton dipole precession frequency is of fundamental importance in NMR.
It is also referred to as the Larmor frequency, and is given by the Larmor
Equation:
o B
where
0
fL
B
=
=
=
=
fL
B
2
2fL= Larmor Frequency (in radians).
Larmor Frequency (in Hertz).
“gyromagnetic ratio”
strength of external magnetic field,
given in Teslas (N.B. 1T = 104 Gauss).
The gyromagnetic ratio, , is defined as the ratio of the magnetic dipole
moment, , to the maximum detectable nuclear spin angular momentum, I of
the material:
I
The value of is unique to each type of nucleus.
Observe from the Larmor equation, that the Larmor frequency, increases with
increasing applied magnetic field, B, i.e. the nuclear dipoles will precess faster in
stronger B fields. Proton dipoles precessing at their Larmor frequency about an
applied magnetic field, form the basis of clinical MRI.
In a given applied B field, different species of nuclei will precess at different
frequencies, since their gyromagnetic ratios are different.
For the hydrogen nucleus (proton), the gyromagnetic ratio is:
where
p
I
2.7928 N
I
N = Nuclear Magneton = 5.05 10-27 J/T
Iproton = ½ ,
=
and
h
6.63 10 34
Js
=
2
2
Substituting these parameters into the equation, we get
proton 2.68 108 Hz/T
10
MRI Physics & Instrumentation Notes
The proton Larmor frequency can now be calculated. For an applied magnetic
field of 1T, we get:
o B
2f B
L
B
f
L
2
Hz
2.68 108
1T
T
2
4.258 10 7 Hz
42.58MHz
Thus, the proton dipole precesses about a 1T magnetic field about forty-two and a
half million times per second!! It is impossible to visualise such a high precession
frequency, but this is how fast things can happen at the atomic level.
Very small variations in B will cause detectably different Larmor frequencies over
a group of identical nuclei. With H-nuclei for example, for B = 0.9T,
fL = 38.3 MHz , and for B = 0.2T, fL = 8.5 MHz. Clinical MR imaging utilises this
fact to allow spatial encoding of body tissue through the application of magnetic
field gradients. (This topic will be dealt with later).
For a group of identical nuclei, the Larmor frequency ( 0 B ) will be the same
for both spin-up and spin-down dipoles. They will all precess about B at the
same frequency, and also in the same direction, as indicated in Figure 3.11.
B
Figure 3.11
11
MRI Physics & Instrumentation Notes
2.2 The radiofrequency (RF) pulse : Its purpose and
application
Recall from Section 1.1, that during the MR imaging process, a Radiofrequency
(RF) electromagnetic pulse is fed into the patient to allow the production and
emission of the NMR signal (from which the final image is constructed). In this
section, we will study the significance of the RF pulse, what it actually does and
how it is produced.
You will remember from your earlier physics studies that the valence electrons
within an atom can be optically excited from one energy level to a higher energy
level if the atom is hit with electromagnetic radiation of the correct frequency (i.e.
within the visible part of the spectrum).
In like manner, the application of an RF pulse to H-nuclei in a magnetic field, serves
to excite some of the excess (10 in 107) spin-up (low energy) protons into the spindown (higher energy) state. By the rules of Quantum theory, such excitation is only
possible if the RF pulse (electromagnetic radiation) deposits an amount of energy
(hf) exactly equal to the energy difference E between the two spin states.
It is a useful exercise to determine what the magnitude of E is, and therefore
what frequency of excitation pulse is required. We determine E as follows:
Figure 3.12 shows an energy level diagram for H-nuclei both in the absence, and
in the presence of an externally applied magnetic field.
spin down
I=-½
E
Eo
(original
energy level
of H nuclei)
spin up
(B = 0) (no mag. field)
I=+½
(B > O)
Figure 3.12
The figure indicates the standard convention, where for protons in a magnetic
field, the spin-down (higher energy) state has the spin quantum number given by
I = ½ and the spin-up (low energy) state has I = +½.
We first determine the energy of the spin-up and spin-down dipoles from the
conventional expression for the potential energy of any magnetic dipole in a B field.
If the magnetic dipole moment is , and it makes an angle with the magnetic field
direction (see Figure 3.13), then the dipole potential energy is given by:
E B
( cos ) B
z B
where z is the component of along the B direction (conventionally called the z- direction).
12
MRI Physics & Instrumentation Notes
B
(z-direction)
Figure 3.13
Students should note that in many MRI textbooks, the -values quoted in
equations are really z. So for example, the dipole potential energy is often
written simply as,
E B .
We can understand the concept of dipole potential energy a little better if we
looked at a bar magnet adjusted to the following orientations in a magnetic field.
Refer to Figure 3.14.
N
E B
S
S
N
E0
S
E B
N
Figure 3.14
When the North pole of the bar magnet is fully aligned with the external field, its
potential to do work is a minimum, given by a negative quantity, (B). When the
magnet is aligned perpendicular to the field direction, its potential energy is slightly
more, this time given by zero. But when the North pole of the magnet is antialigned with the field direction, there is a maximum potential for work to be done if
the magnet is released, and its potential energy is a maximum, given by (B).
Now returning to the nuclear dipole moment, we have that
( z ) I
(since
)
I
So, the dipole potential energy is
E I B
13
MRI Physics & Instrumentation Notes
Hence, for H-nuclei (protons) in the spin-up state (I = +½),
Espin ( 12 ) B
up
B
2
For the spin-down state (I = -½),
Espin ( 12 ) B
down
B
2
Hence the energy difference E between the spin-up and spin-down states is
E E spin E spin
down
up
B B
2 2
B
2
2
Therefore,
E B
We might now expect that if an RF electromagnetic pulse, or photon, with an
energy exactly given by h f = B , were applied to the low energy (spin-up)
magnetic dipoles, they would “jump/flip” into the higher energy(spin-down) state.
This phenomenon is called resonance because the “spin flip” can only occur at a
specific applied pulse frequency for a particular magnetic field strength:
hfphoton = B
Therefore,
fphoton =
=
h
B
We have seen this quantity
earlier, and you will recognise it as being equal
2
to the Larmor precession frequency ( fL) of proton dipoles in a magnetic field of
magnitude, B.
Thus we can see that in order to cause resonance, whereupon proton dipoles are
induced to perform a “spin-flip” from spin-up to spin-down, the necessary
condition is that
Frequency of
applied radiation
(RF pulse)
14
=
Larmor Precession
Frequency of protons
in B-field
MRI Physics & Instrumentation Notes
Thus, for a H-nucleus (proton) dipole precessing in a 1T field at a Larmor
frequency of
fLarmor = 42.58 MHz,
the applied electromagnetic radiation frequency necessary to induce spin-flips is
also
fphoton = 42.58 MHz.
This radiation lies in the radiofrequency (RF) band of the electromagnetic
spectrum (1 kHz to100 MHz).
It is useful to compare the energy of the RF radiation as used in MRI with that of
X-rays. The following calculations show that RF radiation is very much lower in
energy than X-rays.
For a 42.58 Mhz RF pulse,
E photon hf 6.63 1034 Js 42.58 106 Hz
= 2.82 10-26 J.
For a 124 keV X-ray beam,
E photon 124 10 3 1.6 10 19 J
= 1.98 10 -14 J.
Hence,
Ex-ray = (7 1011) ERF pulse
Therefore, RF pulses as used in MRI, are of the order of a million million times
lower in energy than X-rays. They are classed as non-ionising radiation, and are
very much safer than X- rays.
2.3 The magnetisation vector & its manipulation in MRI
What happens following RF excitation (resonance) is of paramount importance in
the MR imaging process. Here, the excited protons gradually start to relax back
to the low energy state. The time for such relaxation varies with tissue-type. This
very useful fact provides a way of differentiating one type of soft tissue from
another. The question is how these different tissue relaxation times might be
observed.
Unlike what occurs in normal optical de-excitation ( where photons are released
from the de-exciting atom), it is found that proton dipoles relaxing back to the
low energy state do not emit RF photons as might be expected. Instead, the
originally absorbed energy B is released and dissipated into the lattice
structure surrounding excited protons, in the form of heat energy.(Such transitions
are called “radiationless transitions”).
The problem now is that if no radiation is actually emitted during de-excitation, how
do we produce a signal from which an MR image could be derived? We need to
first perform a few special tricks before a useful signal can be generated.
15
MRI Physics & Instrumentation Notes
To proceed, we must first define a quantity called the Magnetisation Vector, M.
Consider any normal-sized sample of body tissue in which there exist millions of
H-nuclei. You will recall that when such a sample is placed in an external B-field,
there will be very marginally more spin-up than spin-down nuclei, and all these
nuclei (both spin-up and spin-down) will be precessing about the B direction at
the Larmor frequency.
However the proton dipole moments will be found precessing out of phase with each
other, so that at any instant they would trace out cones as shown in Figure 3.15.
Z
Z
(B)
( B)
(excess spin up)
(spin up)
Y
Y
(spin down)
X
X
Figure 3.15
Each precessing vector (magnetic dipole moment) will have a vertical
component (along the z (B ) direction) and a horizontal component (in the xy
plane). You can see from the diagram that the horizontal components will
collectively cancel out to a zero net value (since the millions of -vectors will be
precessing out of phase). The vertical components will also nearly completely
cancel, except for those due to the excess spin-up dipoles. The vector sum of
these will give a single vector pointing in the +z ( B ) direction. This vector is
called the Magnetisation Vector, M, as illustrated in Figure 3.16.
Z
( B)
Z
(excess
spin up)
M
Y
X
Y
X
Figure 3.16
16
( B)
MRI Physics & Instrumentation Notes
Thus, when a patient is put into an MRI magnet he/she acquires a net
magnetisation vector M pointing in the + B direction. The patient effectively
becomes a weakly magnetised object.
It is the M -vector that is manipulated to produce the final NMR signal. In order to
generate this signal, it is first necessary to move the M vector away from the B
direction. So long as M points exactly along the B direction, it cannot be used to
generate an NMR signal.
When M is parallel to B, it is referred to as the Longitudinal Magnetisation
Vector, (MZ). When M is parallel to B, it cannot precess about B (even though
the individual dipole moments producing M, do!). If M does not precess, no
signal is possible. However, when M is displaced from B, it starts to precess
about B, and an NMR signal can be obtained. How may this displacement be
achieved?
To displace M from B, we apply a separate magnetic field, B1 at right angles to B.
See Figure 3.17. Field B1 is usually orders of magnitude less than B. However it
can still appreciably displace M from B if it has the special property that it
effectively rotates about B at the Larmor frequency ( = B).
Z B
M
Y
o B1
X
Figure 3.17
By applying B1 in this way, M will get pulled away from B, and will try to start
precessing about the direction of the net field ( B + B1 ). However, because B1 is
rotating about B at the Larmor frequency, = B, the net effect is that it will
gradually pull M down and around ( this action may be compared to providing
horizontal flick to a precessing top once every revolution (i.e. at the precession
frequency, o) so as to pull the spin axis down.
Therefore, we apply a “rotating” B1 perpendicular to B, which results in M moving
down and around in a spiral path. This is illustrated in Figure 3.18. If B1 is
switched off after the correct time interval, M can be made to end up precessing
about B in the XY plane.
17
MRI Physics & Instrumentation Notes
B
original M
o
Y
final M (= M xy)
o
B1
X
Figure 3.18
As soon as B1 is switched off, MXY will start to relax back to the B direction,
(also in a spiral motion).
MR Imaging is based on observing the time for relaxation. Different tissue types
have different relaxation times, and these differences are utilised in producing
tissue contrast in the final MR image. This will be discussed in detail later.
The motion of the M vector away from the z-axis after applying the (rotating) B1
field, would be easier to visualise by changing our frame of reference. For
example let us imagine ourselves to be an observer circling about the z-axis in the
XY plane at the same (Larmor) frequency as the M and B1 vectors (for example,
think of an observer sitting on the rotating B1 vector). From this perspective, M
will not be seen to spiral about B (so the observer will conclude that B does not
exist). Instead, M will only be seen to be in the presence of B1 , and so to precess
about B1 at frequency given by
1 = B1.
This is illustrated in Figure 3.19 in which the rotating B1 is located along the X’
axis in the reference frame rotating at the Larmor frequency. To an observer
stationary in this frame, M will be seen to precess in a vertical circle as shown at
frequency 1.
Z
Rotary reference
frame: (observer
doesn’t see B, and
sees B1 stationary)
M
(before B1
applied)
1 B1
Y’
B1
M will be observed to
precess about B1 in
the vertical circle
shown, at 1 = B1
X’
Figure 3.19
18
MRI Physics & Instrumentation Notes
If the rotating B1 field is turned off just as the M vector is pulled into the XY plane,
then MXY being only in the presence of the applied magnetic field B , will start to
precess about B in the XY plane at a frequency o = B, before relaxing back to
its vertical position via a spiral path.
We call the application of the B1 field used to pull M perpendicular to B (i.e. to
convert MZ to MXY), a “90 pulse”. The 90°pulse is the RF pulse referred to
earlier, and its production will be discussed in the next section. A summary of the
sequence of events is illustrated in Figure 3.20.
Z
B
Z
Apply
90 0 pulse
Mz
B
In time,
spiral path
takes vector
back to vertical
M XY
Y
Y
oB
(precession in XY
plane immediately
after pulse)
X
Figure 3.20
Once M has been pulled into the XY plane, there can be no component of the
magnetisation vector remaining in the B direction. That is, MZ = 0. When this
condition exists, there must be an exact balance between the spin-up and spindown dipole populations. That is, no excess spin-up dipoles can exist. For
example, if originally there are an excess of 4 1016 spin-up dipoles in a
hypothetical sample in a magnetic field, then the 90 pulse must supply
resonance energy to flip half (2 1016) of these dipoles into the spin down
(higher energy) state.
The MXY vector is called the transverse magnetisation vector, since it is the M
vector that has been pulled through 90°. Immediately following the 90 pulse, the
magnitudes of the transverse and longitudinal magnetisation vectors are the same,
i.e. MXY Mz .
The fact that a coherent vector, MXY , exists, and precesses in the horizontal
XY- plane, implies that the equally populated spin-up and spin-down dipoles must
all initially precess in phase. This is illustrated in Figure 3.21.
19
MRI Physics & Instrumentation Notes
B
z
Excess spin-up
dipoles
(B)
Mz
Mxy
Y
Y
90° Pulse
X
X
Excess spin-down
dipoles
Figure 3.21
From these observations, we can therefore see that the 90 pulse does two things:
i. it brings about equally populated energy states amongst the proton dipoles, and
ii. it causes all proton dipoles to precess about B in phase initially.
In MRI, we observe two independent relaxation processes following the 90
pulse:
i.
the decay of MXY to zero, which is called, “Transverse Relaxation”; and
ii.
the re-growth of MZ to its original (maximum) value, which is known as
“Longitudinal Relaxation”.
We will discuss these different relaxation processes in some detail later.
2.4 The production of the 90 pulse
In practice, the 90°RF pulse is not produced by actually rotating the B1 field ( as
suggested in the last section). Instead, use is made of a B1 field which is made to
oscillate in magnitude along the x-direction (i.e. perpendicular to the applied
magnetic field B). This action is easier to perform, and has same net effect on M
as a rotating B1 field would have.
An AC current of appropriate RF frequency is passed through a metallic coil
whose axis lies along the x-axis. See Figure 3.22. This AC current produces an
oscillating B1 field (along the x-axis) of the same RF frequency. By setting the
AC frequency to match the proton Larmor frequency, the MZ vector will
commence to precess about B and spiral towards the XY- plane (and possibly
beyond). An observer rotating at the Larmor frequency in the XY plane will
simply see MZ fall along a vertical circular path as shown in Figure 3.22.
20
MRI Physics & Instrumentation Notes
B
(Z)
Simple are mation of Mz as viewed in
rotating reference frame (, = B1)
MZ
Y
MXY
“transmitter”
coil
(or RF Coil)
B1
X
~
RF Oscillator
(set to Larmor freqency)
Figure 3.22
The duration of the RF pulse determines by how much MZ will effectively get
pulled down. Remember that MZ will effectively precess about the B1 field, in the
vertical (YZ) plane at a frequency 1 = B1 . Therefore,
2
B1
T1
where T1 is the time for 1 full revolution of MZ in the vertical plane.
The duration of a 90 pulse must be a quarter revolution or T 1 .
4
So we have
seconds.
2 B1
90 pulse duration =
Note that the pulse duration is inversely proportional to B1, as expected.
2.5 Emission of NMR signal & free induction decay (FID)
As we know, following the 90 pulse, the transverse magnetisation vector, MXY
precesses about B in the XY plane at the Larmor frequency (0 = B ). As MXY
rotates, it will produce an alternating magnetic flux () in the metallic coil that
originally produced B1 . This, in turn, will produce an alternating induced emf
() in the coil. If the coil has N turns, we have from Faraday’s Law:
N
d
dt
(where and vary at the Larmor frequency, o).
21
MRI Physics & Instrumentation Notes
Thus the coil now behaves as a receiver and the induced emf (also of frequency
o) is the radiofrequency NMR signal “emitted” from the tissue sample being
studied. This signal generated in the pick-up coil may be observed on a Cathode
Ray Oscilloscope or other detector, as illustrated in Figure 3.23.
Z
B
Y
MXY
MR Signal
Pick-up Coil
X
Key
~
CRO or
other
detector
Figure 3.23
Following the 90 pulse, the transverse magnetisation vector MXY starts to decay
in magnitude, as the system (tissue sample) returns to “thermal equilibrium”
through dipole dephasing (transversal relaxation). Consequently, the NMR signal
in the pick-up coil also decays in amplitude as shown in Figure 3.24.
90o Pulse
(induced
emf)
rate of decay of MXY
time
Figure 3.24
This signal is known as the F.I.D. (“Free Induction Decay”) due to the fact that it
is produced by electromagnetic induction in the pick-up coil, and it decays freely
as MXY decays to zero. The envelope of the FID reveals the rate of MXY decay,
i.e. the rate of transversal relaxation.
We will now proceed to study Transverse and Longitudinal Relaxation separately.
22
MRI Physics & Instrumentation Notes
2.6 Transverse relaxation (or “Spin-spin” or “T2”
relaxation)
Remember - immediately after the 90 pulse, the (excess) spin-up and spin-down
dipoles precess about the applied B field in phase with each other (and MXY
precesses about B in the XY plane - refer to Figure 3.21). The precession
frequency is 0 = B. Now, for the dipoles to continue to precess at identical
frequencies, and hence remain in phase, the magnetic field at the site of the Hnuclei (protons) must be absolutely uniform. However, this is impossible to
achieve as no magnet has a perfectly homogeneous B field, and the very act of
immersing a patient into the applied magnetic field, changes its uniformity
somewhat. However, for discussion, let’s initially assume that the applied B is
uniform. Then one would expect that all proton dipoles throughout the imaged
tissue sample would precess at exactly the same Larmor frequency (0 = B)
following the 90 pulse, and so remain aligned forever.
This, however, does not happen! In any sample of tissue, there will be millions of
charged particles spinning and rotating (e.g. orbital electrons). These create their
own variable local magnetic fields in the vicinity of the precessing proton
dipoles. Different net magnetic fields will be therefore be experienced by each
precessing dipole at different stages within its precession cycle (i.e. as a proton
dipole moves towards and then away from other moving charges). The net
magnetic field is the vector sum of the applied B field and the local “spin” field.
The local magnetic field variations are completely random. Consequently it is
impossible to predict when a precessing dipole will experience an increase or
decrease in local (and hence net) magnetic field.
Although the variable local fields are orders of magnitude less than the applied
field B, their effect is important: They cause de-phasing of the precessing dipoles
due to random variations in the precession frequencies (since 0 = Bnet , and
Bnet is intrinsically variable). This dephasing process is illustrated in Figure 3.25.
Z
Z
Z
(only spin-up
dipoles drawnfor convenience)
Y
Y
M XY
X
Y
M XY
M XY
X
X
Top
View
Figure 3.25
Observe from this figure that as the dephasing process continues, the dipoles fanout and MXY shrinks in magnitude from its maximum value following the 90
23
MRI Physics & Instrumentation Notes
pulse. This transversal relaxation is revealed by the “emitted” RF signal whose
amplitude is directly proportional to MXY, as illustrated in Figure 3.26.
M0
0.37 M0
MXY = M0e -t/T2
90o Pulse
Net MXY
or signal
strength
T2
time
(Assumes perfectly homogenous applied B field)
Figure 3.26
Hypothetically assuming a perfectly homogeneous external magnetic field B, the
rate of decay of MXY is given by the expression
MXY = Mo e- t/T2
where M0 is the original transverse magnetisation (following the 90 pulse), and
the quantity T2 , known as the “transverse relaxation time constant’, or more
commonly, the “ T2 relaxation time” , is the time for MXY to reduce to 1 e .Mo ,
i.e. the time for MXY to drop to (0.37) of its original (maximum) value. It takes a
timespan of about (4.6 T2) for MXY to decay to 1% of its original value, Mo.
Transverse relaxation is called “spin-spin” relaxation (due to the interaction of
spinning dipoles with local magnetic fields produced by other spinning
neighbours). It is also known as “T2” relaxation.
Different soft-tissues in the body are found to possess quite different T2
relaxation times. The T2 relaxation times are generally longer for tissues with
higher water content, e.g. inflamed, edematous and malignant tissues. This is
because the structure of the water molecule is such that the hydrogen protons in
each molecule are relatively far apart and spin-spin interactions between them
relatively small. Fats and lipids generally have shorter T2’s. Solids have the
shortest T2 because their highly compact structure allows intense spin-spin
interactions between closely neighbouring protons and nuclei. We will see later
how we measure T2 .
2.7 T2 Relaxation (pronounced “ tee-two star” )
The above discussion of T2 has assumed that the applied magnetic field B is
perfectly homogeneous, and so has assumed that the decay of MXY is purely due
to dephasing from “spin-spin” interactions. However, the applied magnetic field
B is not perfectly homogeneous, and variations in B are difficult to eliminate.
24
MRI Physics & Instrumentation Notes
These macroscopic (or static ) field variations are usually much larger than those
due to electron and nuclear spin. There will be some portions of examined tissue
lying in a higher applied field than neighbouring portions. Consequently,
precession speeds will vary with location even within small imaged regions,
causing rapid dephasing of proton dipole moments after a 90 pulse. Such
dephasing is much more rapid than if only due to spin-spin interactions.
Hence in real situations, the emitted signal (FID) is observed to decay much more
rapidly than described by T2. The net decay of MXY will be due to a combination
of static fluctuations in the applied magnetic field B and the random fluctuations
in the local “spin” magnetic field originating within the tissue. The observed
decay of MXY (i.e. the real FID signal), is described by the time-constant T2*
rather than T2: so the decay equation is:
MXY = Moe- t / T2*
where
T2* =
M0 =
actual time for MXY to drop to 0.37 Mo
original MXY at time of 90 pulse.
Figure 3.27 illustrates the relaxation of MXY and the decay in the emitted signal
strength with both a perfectly homogeneous magnet and a real (inhomogeneous)
magnet.
Net Mxy
or signal
strength
90o Pulse
signal decay (T2) with perfectly homogeneous magnet
signal decay (T2*) with real (inhomogeneous) magnet
time
(real) FID
Figure 3.27
It can be seen that T2* << T2. For most soft tissues, T2 is of the order of between
50 and 350 ms and T2* a few ms.
So summarising,
T2* decay represents proton dipole dephasing in response to inhomogeneity in the
applied B field , whilst T2 decay represents proton dipole dephasing purely due to
spin-spin interactions (through the assumption of a perfectly homogeneous
external B). The magnitude of T2 is characteristic of the tissue material under
investigation.
25
MRI Physics & Instrumentation Notes
Before discussing exactly how T2 is determined for different tissues (given that
what is directly measured is only T2 ), we will first digress to describe the other
important relaxation process occurring in MRI, namely Longitudinal Relaxation.
2.8 Longitudinal Relaxation (or “Spin-lattice” or “T1”
relaxation)
You will recall that application of the 90 pulse makes the longitudinal
magnetisation vector, MZ = 0, so that half of the excess spin-up dipoles will have
been excited into spin-down state.
Following the 90 pulse, these excited dipoles gradually “flip back” or de-excite to
the low energy, spin-up state. During de-excitation, the absorbed energy is
released and dissipated into the local environment or “lattice”, as heat energy . MZ
gets re-established. This regrowth is an exponential process, defined by a timeconstant, T1, as illustrated in Figure 3.28.
90o Pulse
MZ
t=0
Mz at equilibrium ( = Mo)
T1
2T1
3T1
4T1
time
Figure 3.28
This regrowth curve can be described by the equation
MZ = M0 (1 - e- t/T1)
where,
MZ = instantaneous value of the longitudinal magnetisation vector at time t
following the 90 pulse,
M0 = final value of the longitudinal magnetisation,
and
T1 = spin-lattice relaxation time constant (usually referred to as the “spin-lattice
relaxation time”).
When t = T1,
MZ = M0 (1 - 1e )
= 0.63 M0
i.e. T1 is the time interval for 63% regrowth of Mz. It takes approximately (4.6
T1) for 99% regrowth of Mz.
26
MRI Physics & Instrumentation Notes
It should be noted that T1 will vary with the environment the H-nuclei (protons)
find themselves in, i.e. with tissue-type. Also, T1 cannot be directly measured (as
T2* could from the FID signal), since the de-excitation energy is dissipated as
heat in the lattice, and this energy is unable to be harnessed to produce a
detectable signal.
It is also very important to note that the time interval for complete de-excitation ,
i.e. full regrowth of MZ, is not the equal to the time for full decay of MXY (i.e.
complete dephasing of dipoles), following the 90 pulse. These processes of
dephasing and de-excitation are completely independent (T1 T2). Usually,
dephasing is nearly always completed before full thermal equilibrium (i.e.
complete regrowth of MZ) is established: i.e. T1 T2.
The magnitudes of T1 and T2 and their difference vary, depending on tissue type.
For example, in liquids, T1 T2 . For cerebral spinal fluid (CSF) in an external
magnetic field of B = 1T, T1 = 3000 ms and T2 = 2000 ms. On the other hand, in
muscle,T1 >> T2. In this case, for B = 1T , T1 = 750 ms and T2 = 55 ms.
Generally, the more water in a tissue, the larger T1, whilst the greater the
concentration of proteins, medium-sized macromolecules and lipids, the shorter
T1. For example, in a 1T applied field, T1 ~ 200ms for fat and ~ 390ms for white
matter of the brain (the latter having a high lipid content). Tissues which are
inflamed, edematous, or malignant have a higher water content than their
surrounding tissue medium, and so have a larger T1 than normal. Images showing
these T1 differences can reveal pathology.
The large variation in T1 with tissue type, prompts the question as to why the deexcitation times vary with lattice environment. This question can be answered in
the following way. The excess proton dipoles precessing in the spin-down (high
energy) state following a 90 pulse, will be able to de-excite (and release energy to
the lattice) quickly (i.e. T1 will be short), if the lattice molecules to which the
hydrogen protons are connected, or are proximal to, translate, rotate, or vibrate at
a frequency comparable to the proton Larmor frequency (o).In this situation, the
local magnetic field fluctuations within the lattice are predominantly of similar
frequency to the proton Larmor frequency. On the other hand, when the lattice
molecules translate, rotate or vibrate too slowly compared with the proton Larmor
frequency (for example, as in solids, and in tissues with many very large
macromolecules), or too fast (as in pure liquid /water), T1 is long, since the
protons are now unable to efficiently hand over their energy to the lattice. These
variations in T1 with medium /tissue type, together with corresponding T2
variations, are illustrated in Figure 3.29.
time
T1
T2
T1 minimum when local fields fluctuate at ~ proton Larmor
frequency (tissues with medium sized biomolecules) T1
long for both solids/tissues with very large macromolecules, and for pure liquids/water (very small fast
moving molecules)
T1
slow
(solids, etc.)
fast
(pure liquids)
Figure 3.29
27
MRI Physics & Instrumentation Notes
Figure 3.29 shows that T1 is a minimum for tissues with medium-sized
biomolecules. Examples of tissues with short T1 are fat, white matter and
proteinaceous fluids. Here, the local fields fluctuate at frequencies of similar
magnitude to the proton Larmor frequency, and energy transfer from the dipoles to
the lattice is efficient. This is not the case for solids and pure liquids for which T1
is long. Grey matter acts like a typical solid tissue; with less fat than white matter,
it has intermediate T1. Urine and CSF have long T1. Figure 3.29 also shows the
trend for T2. The graph shows that for any tissue material, T2 is less than T1; for
solids T2 is very short, and for pure liquids it is very long.
It should also be noted that T1 has a dependence on magnetic field strength. It is
found to get longer in stronger applied B fields. This is because with increasing
B there is a larger energy difference between the spin-up and spin-down states (
E = B), and so energy transfer to the lattice during de-excitation is more
difficult. Unlike T1, the transverse relaxation time, T2 is much less sensitive to
the strength of the applied magnetic field B .
2.9 Spin density
Magnetic resonance imaging is primarily based on T1 and T2 variations between
different types of soft tissue. However there is also a third parameter called spin
density which may be used to produce images.
Spin density is defined as the number of spinning protons per unit volume in a
sample of tissue. The number of protons per unit volume will affect the size of
the magnetisation vector, M. Clearly, the greater the proton (spin) density, the
greater the number of excess spin-up dipoles, and the larger M will be.
Consequently, following application of a 90 pulse, both the transverse
magnetisation MXY, and the FID signal will be of larger amplitude if the tissue
spin density is high . We can utilise the relative variations in FID amplitude to
differentiate between tissue-types. It must be ensured that the tissue sample being
examined is in a state of thermal equilibrium before application of a 90 pulse, in
order to avoid a false spin density indication. For example, if complete deexcitation (regrowth of MZ ) has not yet occurred following a 90 pulse, then
premature re-application of another 90 pulse will produce a smaller FID signal,
giving the impression that the tissue is of lower spin density.
There will be more discussion on spin-density in Section 3.2.12, where it will be
seen that there are special cases where the existence of H-nuclei does not always
imply a large detected signal. This section will also look at the requirements for
images which display spin-density variations.
2.10 Measurement of T2, & T2-weighted images
We have seen that T2* and Spin Density information can be got directly from the
FID signal. However, T2 and T1 information cannot. How then, are these
parameters measured ?
28
MRI Physics & Instrumentation Notes
We use “pulse sequences” to determine T2 and T1. These are groupings of two
or more RF pulses that may be applied in different combinations to body tissue to
obtain an MR image. Several types of pulse-sequence exist in MR imaging.
However we will only consider two simple sequences in this introductory MRI
module. These include a series of 90 pulses or several groups of 90 and “180“
pulses applied a fixed time-interval apart, to body-tissue during the relaxation
processes. We will start with a description of one of these simple pulse sequences
which may be used to determine T2. In the next section, we will discuss the other
simple sequence when talking about T1 measurements.
Recall that T2 relaxation is the dephasing of dipoles precessing in the XY plane
(following a 90 pulse) due entirely to local magnetic field fluctuations. The
observed FID signal shows T2* relaxation which includes the effect of the
permanent, unavoidable B -field inhomogeneity of the MRI magnet.
We apply a “spin-echo” pulse sequence to remove the effect of the applied B field inhomogenity, and hence to show up T2 (spin-spin) relaxation within the
observed tissue. Note that the rate of dipole dephasing is more severely affected
by the relatively large static variations in the applied B field, than by random
local B fluctuations. Despite this fact, dephasing resulting from the applied field
inhomogeneities can be effectively annulled (i.e. compensated for), whilst
dephasing from local random B fluctuations cannot. This compensation will
allow true T2 dephasing to be measured. To understand how this is possible, let
us first study the following simple analogies:
Analogy (i)
Suppose we have 4 athletes of approximately equal ability taking part in a foot
race around a circular track. Suppose they start together (in phase) and run at
slightly varying speeds. Their speed changes will be random - individually, the
runners will speed up, overtake, slow down, fall behind at random, and eventually
become scattered along a section of track. Now assume a “giant hand” plucks all
the runners simultaneously off the track and places them on other side of the trackaxis as depicted in Figure 3.30.
later
start
track
axis
still
later
after
“pluck”
Figure 3.30
29
MRI Physics & Instrumentation Notes
After the “giant hand pluck” the runners continue to run at random speeds and
merely get more scattered along the track as time progresses, never again
getting back in phase with each other ( because the runners are of approximately
equal ability).
Analogy (ii) :
Now suppose we have 4 runners of very different abilities taking part in the race an Olympian, a very good amateur, a weekend jogger and grandpa (with bunions).
They start in phase, but very quickly get out of phase. If the same “giant hand”
simultaneously plucked all the runners and set them down on the opposite side of
the track axis, then the fastest (Olympian) would be furthest behind and slowest
(grandpa) in front, as illustrated in Figure 3.31.
(slowest)
later
(fastest)
start
track axis
(fastest)
still later
after “pluck”
(slowest)
Figure 3.31
In reasonably quick time, however, the runners would start to “bunch-up” again.
If the time interval from the start of the race to the “giant hand pluck” is equal to
(tau), then assuming the runners maintain their individual average speeds, they
would “bunch-up” or “ re-phase” again at t = 2. However, this rephasing could
never be as good as it was at the start of the race, due to random speed variations.
Immediately following the rephasing, the runners will very quickly start to get out
of phase again, as before.
Analogy (i) is equivalent to the gradual dephasing of dipoles due to random local
field variations alone, and their inability to re-phase. Analogy (ii) is equivalent to
the rapid dephasing of the precessing dipoles due to the larger, static variations in
the applied field B, and their amenability to substantial re-phasing.
The “giant-hand” trick in both analogies above is equivalent in MRI, to what is
known as a “180 RF pulse”. The purpose of this RF pulse is to produce dipole
rephasing following a 90 pulse, and its operation will be described below. The
repeated application of a combination of a 90 pulse followed by a 180 pulse is
very useful in MRI, and is known as a “spin-echo” pulse sequence. It is a
30
MRI Physics & Instrumentation Notes
workhorse amongst pulse sequences, and can be used to obtain T2-, T1- and spindensity weighted images.
Let us examine a (90 - 180) pulse sequence to see how the 180 pulse achieves its
purpose, and how this sequence leads to the measurement of T2 relaxation times.
The effect of applying a 90 pulse, followed after a certain time-interval by a 180
pulse may be understood from Figure 3.32.
Z
M
Z
90
Z
o
180
time
f
Y
Y
time
M XY
M XY
S
f
S
Y
MY
X
Z
o
pulse
pulse
X
Z
Y
M XY
X
Vertical components of
individual excess spinup dipole moments
X
X
M XY has grown
after rephasing
Figure 3.32
As we know, the magnetisation vector M (composed of the vertical components of
the excess spin-up dipole moments) initially points in the +Z (applied field B)direction. The 90 pulse pulls M and the “z -components” of individual proton
magnetisation’s (dipole moments) into the XY plane. These vectors start to
precess about B in the XY plane, and quickly get out of phase, with some moving
faster than others. The transverse magnetisation vector, MXY therefore rapidly
decreases in magnitude.
A 180 pulse is now applied to the tissue sample at a selected time interval (tau)
following the 90 pulse. The 180 RF pulse is often produced in the same way as
the 90 pulse, through the application of the same alternating magnetic field, B1
(oscillating in the x-direction at the Larmor frequency), but for double the
duration of the 90 pulse. Alternatively, the 180 pulse may be produced by
choosing an RF burst kept on for the same duration as the 90 pulse, but
possessing twice the strength (power) of the 90 pulse. The 180 pulse has the
net effect of rotating MXY, and hence the individual horizontal components of the
precessing dipole moments, through 180 to a mirror-image position in the XY
plane. (As in an earlier discussion in Section 2.3, it is easier to visualise this
motion if the observer were in a rotating coordinate system similar to that depicted
in Figure 3.19, i.e. if the observer was rotating in the XY plane at the Larmor
frequency. In this case, the observer would not see MXY precessing about the
applied field B, but would only see it move in a vertical circle about B1). As
depicted in Figure 3.32, the 180 rotation of the individual dipole components in
the XY plane, makes the originally fast (f) dipoles now lag the originally slow (s)
ones, similar to what happened with the runners after the “giant pluck” in
31
MRI Physics & Instrumentation Notes
Analogy (ii). Now, after another time interval following the 180 pulse, the fast
dipoles will, to a good extent, have caught up (i.e. rephased) with the slow ones,
and MXY will have grown to a maximum value (equal to that expected if the
applied field B was perfectly homogeneous).
However, due to the on-going dephasing effect of the random local field
fluctuations (from spin-spin interactions) MXY is unable to re-grow to its original
maximum value found immediately after the 90 pulse. Immediately after the
induced rephasing of the transverse dipole moments, the dipoles begin to dephase
again.
The changes in MXY during the pulse-sequence are used to produce a pattern of
emitted RF signals, from which T2 can be measured, and MR images obtained.
After the 90 pulse, the FID signal decays rapidly due to T2*. But following the
180 pulse, MXY, and thus the detected signal, regrows to a maximum, before T2*
dephasing again reduces the signal amplitude. This re-growth and immediate
subsequent decay of the detected signal is called the “spin-echo”. The peak of the
spin-echo signal is always less than the peak at the beginning of the FID signal,
and will be diminished by exactly the amount of true spin-spin dephasing (T2
relaxation): This is illustrated in Figure 3.33.
T2*
180 o Pulse
Mxy or
signal
strength
90 o Pulse
T2 decay
spin-echo
T2*
t=0
t =
t = 2
t = 3
time
Figure 3.33
It is possible to add yet more 180 pulses to the above sequence. Suppose one
were applied at time t=3 (in Figure 3.33). Then the proton dipoles dephased by
the applied magnetic field inhomogeneities will undergo a reversal, with fast
dipoles lagging and slow ones leading. Rephasing of the fast and slow proton
dipole moments would yield another spin-echo signal. The amplitude of this
however, would be even smaller than the first spin-echo: this is so because, as
already mentioned, the random dephasing caused by true tissue T2 relaxation
processes continue the whole time; and while non-random dephasing (due to fixed
variations in applied B) can be rephased, T2 de-phasing due to tissue itself cannot.
The T2 relaxation curve is therefore that which connects the peak of the FID and
those of the subsequent spin-echoes produced by successive re-application of 180
pulses. This is illustrated in Figures 3.33 and 3.34. Each successive spin-echo
helps in the mapping of the T2 relaxation curve. In addition, each spin-echo may
be used to produce a separate MR image ( these images will have different
degrees of T2 contrast dependence, as discussed below). The time that elapses
between the 90 pulse and the first spin-echo is called the “time-to-echo” or “TE”
32
MRI Physics & Instrumentation Notes
as shown in Figure 3.34. This time interval is equal to twice the time between the
90 and the 180 pulses (i.e. TE = 2). In a more general sense, TE is also taken
as the time from the initial 90 pulse to any subsequent spin-echo in the sequence.
These TE’s will then be designated as TE1, TE2, etc. TE is a very important
parameter in MR imaging, and is one that can be varied by the operator. Its
significance will be discussed below.
Mxy or
signal
strength
90° Pulse
T2 decay
spin-echoes
T2*
180°
pulse
time
180°
pulse
TE
Figure 3.34
Let us now look at the situation where we have two tissues with different T2
relaxation times. By applying a spin-echo sequence, these T2 differences can be
observed. Figure 3.35 shows the T2 relaxation curves for Cerebrospinal Fluid
(CSF) and White Matter of the Brain. A large spin-echo amplitude is obtained for
CSF with a long T2, and a smaller spin-echo signal is obtained for White Matter
with a shorter T2. The diagram shows the spin-echoes obtained when both a short
and a long TE is applied by the operator.
Long TE
or
Sig
nal
XY
M
T2 relaxat
small
signal
difference
n
larger
signal
difference
spin echoes
Pul
se
o
90
CSF (Long T2)
FID
White Matter of Brain (Short T2)
Short
TE
TE 1
(TE 1 < TE 2 )
TE 2
Figure 3.35
For very short TE (~ 15-25 ms), the spin-echo amplitudes are large for both
tissues, but their amplitude difference is small. The effect of applying such short
TE is that an MR image based on T2 differences will show poor contrast
33
MRI Physics & Instrumentation Notes
between these tissues. For long TE (~ 60-130 ms), the spin-echo signals have a
bigger amplitude difference, so that image contrast is more pronounced. However
this gain in contrast is at the expense of signal strength - image “noise” tends to
increase with increasing TE, and spatial resolution is reduced. Application of a very
long TE should produce the best tissue contrast, but the spin-echo amplitudes will be
very tiny and the signal-to-noise ratio is too small to be useful.
So, through the correct choice of TE, tissues with long T2 will give a strong spinecho signal and will appear bright in the image, whilst tissues with short TE will
appear dark. Such an image, whose contrast is produced essentially by T2
differences is called a “T2-weighted” image.
Figure 3.36 shows a typical T2 weighted image of the brain. Here, cerebrospinal
fluid, having a relatively long T2, appears bright; the substance of the brain,
especially the white matter, appears dark because of its shorter T2.
Figure 3.36
Source: Westbrook, Catherine (1999) Handbook of MRI Technique
We should note that if other types of MR image are desired, which “block out”
T2 differences (such as “T1 weighted” images, to be discussed presently), then a
very short TE must be used. In this case, negligible T2 contrast will be seen.
2.11 Measurement of T1, & T1-weighted images
Recall that T1 (spin-lattice) relaxation is the re-growth of the longitudinal
magnetisation vector MZ, following a 90 pulse. After some time, thermal
equilibrium is re-established and MZ reaches its original (and maximum) value,
M0 as shown in Figure 3.37. T1 is the time for 63% regrowth of MZ, and
different tissues have different T1 values.
34
MRI Physics & Instrumentation Notes
Mz
Mo
90
o
Pulse
M z =0.63Mo
time
T1
Figure 3.37
The regrowth of MZ is a “silent” event - it does not produce an RF signal, unlike
MXY. So, to produce an image that reflects T1 relaxation, we must apply a pulse
sequence which generates an RF signal proportional to MZ.
In principle, the simplest pulse sequence to show T1 relaxation is a series of 90
pulses. This is known as the Saturation Recovery sequence, and symbolised as
(90 - 90 - 90 -). The time interval between 90 pulses is called the “time to
repetition” or “TR”. The choice of this interval is important and like TE, can be
controlled by the operator of the MRI machine.
If TR is made very long, so that longitudinal relaxation is complete before reapplication of the next 90 pulse, then when the latter pulse is applied,
|MXY| = |M0| and the FID (RF) signal produced will have maximum amplitude.
On the other hand, if TR is reduced, such that MZ is still growing when the 90
pulse is re-applied, then a smaller MXY, and hence FID signal will be produced.
The shorter TR, the smaller the FID (RF) signal. Hence the FID amplitude is
directly proportional to the degree of T1 relaxation at time, TR.
Figures 3.38. and 3.39 illustrate the situations for TR T1, and TR T1,
respectively.
Longitudinal Relaxation
90 o Pulse
M XY (=M 0 )
FID
[TR >> T1]
TR
FID
time
90 o pulse
90 o pulse
90 o pulse
Figure 3.38
35
MRI Physics & Instrumentation Notes
Mz
90o Pulse
Mz (< Mo)
[TR T1]
TR
FID
time
90o pulses
Figure 3.39
Now suppose that we have two kinds of tissue being simultaneously imaged.
Suppose one kind has a long T1 and the other a short T1. On application of the
90 pulses, the two tissue types will have different MZ values, and hence different
MXY and different FID signal amplitudes. This is illustrated in Figure 3.40.
90o Pulse
longitudinal magnetization
short T1
tissue
FIDs
long T1
tissue
time
TR
90o
pulse
90o
pulse
Figure 3.40
From Figure 3.40, it can be clearly seen that a tissue with a short T1 will produce
a large amplitude FID signal, whilst that with a long T1 will yield a small
amplitude signal. Therefore an MR image constructed from these signals would
display the tissue with short T1 as bright, and that with the long T1 as dark. It
should be noted that if TR ( as set by the operator) is made very long ( say
~2000 ms), then both tissues will have reached complete T1 relaxation between
90 pulses, and no T1 difference would be seen in the FID amplitudes. This is
shown in Figure 3.41.
“short”
TR
short T1
FIDS (amplitudes of both signals
much the same)
long T1
TR
(long)
t
90o
pulse
o
90 pulse
Figure 3.41
36
MRI Physics & Instrumentation Notes
Hence for T1-dependent images, we must have short TRs (usually ~ 200-800 ms
for common applied B-fields). Figure 3.42 shows a “T1-weighted” image of the
head, where most of the contrast depends on the T1 variations among tissues.
Tissues with long T1 values(e.g. cerebrospinal fluid ) appear dark and tissues
with short T1 (e.g. white-matter of brain) values appear bright.
Figure 3.42
Source: Westbrook, Catherine (1999) Handbook of MRI Technique
The 90 pulse sequence that we have described shows in principle how T1weighted images might be derived. However this sequence is somewhat
problematic in that the FID signal produced immediately after each 90 pulse is
hard to measure as a distinct signal - it is many orders of magnitude smaller in
amplitude than the 90 pulse itself, and can get “swamped” by this large RF pulse.
Consequently, the (90 - 90 - 90 - ) pulse sequence is not used in practice. The
problem is overcome by using spin-echo sequences to produce T1 dependent
images. As was seen in Section 3.2.10, a spin-echo is formed only some time
after the end of the last applied 180 RF pulse, so that the entire spin-echo signal
can be used to produce an MR image without being overwhelmed by the large RF
pulses ( as is generally the case with the FID signals). One sequence that may be
used is the “90 - (90 -180) - (90 -180) -” sequence. This is to be contrasted to the
spin-echo sequence described in Section 3.2.10 used to produce T2-weighted
images: i.e. the “90 - 180 - 180 - 180 -” sequence. It should be noted that the
“90 - (90 -180) - (90 -180) -” sequence used to show up T1 differences, can also
be used to show T2- and spin-density differences. It all depends on the choice of
TR and TE used, as to whether a T1-, T2-, or spin-density weighted image is
obtained. Let us now have a good look at this spin-echo sequence, as illustrated in
Figure 3.43.
37
MRI Physics & Instrumentation Notes
“90 - (90-180) - (90-180)” sequence:
MXY (= Mo)
90o Pulse
Mz(<Mo)
Mz(<Mo)
T2 relax
T1 relax
original
FID
T1 relax
spin echo
FID
90o
pulse
T2 relax
T1 relax
T2 relax
180o
pulse
90o
pulse
180o
pulse
TE
TR
TR
Figure 3.43
The Figure shows T1 and T2 relaxation curves following the initial 90 pulse.
The time to repetition, TR, has been set so that the second 90 pulse occurs before
T1 relaxation is complete. This produces a smaller transverse magnetisation,
MXY, and hence a smaller FID amplitude, than was produced after the first 90
pulse. T1 and T2 relaxation re-commence after the second 90 pulse and proceed
independently. A 180 pulse is applied shortly after this 90 pulse to rephase the
proton magnetisation’s in the XY plane and to produce a spin-echo at a time TE
after the 90 pulse. The amplitude of the spin-echo will be governed by the size of
MZ (i.e. by the degree of longitudinal relaxation) at the time of the 90 pulse, as
well as on the T2 relaxation rate of the tissue.
Suppose now that we have two tissues of different T1 (one long and one short).
The above pulse sequence is capable of producing different spin-echo amplitudes
for the two tissues, as shown in Figure 3.44.
90o Pulse
MXY=(Mo)
short T1
Mz1
Mz2
long T1
spin
echoes
90o
pulse
90o
pulse
90o
pulse
180o
pulse
TE
TR
Figure 3.44
38
180o
pulse
MRI Physics & Instrumentation Notes
By appropriate choice of TR and TE, the difference in these spin-echo amplitudes
can be made to substantially reflect the differences in MZ at the time of the second
and subsequent 90 pulses, and hence the difference in the T1 relaxation times for
the two tissues. For example, if TE is kept short, then differences due to T2
variations will be small. By also keeping TR relatively short, the difference
between MZ1 and MZ2 for the two tissues at the time of the 90 pulses (see Figure
3.44) is maximised, and the spin-echo amplitude difference will reflect mainly
T1 differences. Hence a large spin-echo amplitude will represent a short T1
tissue, and a small spin-echo will correlate with a long T1 tissue (see also Figure
3.45). The corresponding MR image is called a “T1-weighted” image with the
short T1 tissue appearing bright and the long T1 tissue dark.
spin echoes
short T1
(tissue 1)
T2 relax (tissue 1)
long T1
(tissue 2)
T2 relax (tissue 2)
t
{
TR (short)
TE
(short)
Figure 3.45
2.12 TR and TE choices for T2- and spin-density-weighted
images
The spin-echo sequence just used to show T1 differences can also be used to
display T2 and spin-density weighted images. Let us see how this is done, by
appropriately adjusting TR and TE.
To obtain T2-weighting, we use a long TR and a long TE, as illustrated in
Figure 3.46.
short T1 (tissue 1)
T2 relax. (tissue 1)
long T1
(tissue 2)
T2 relax.
(tissue 2)
spin echoes
90 o
pulse
90 o
pulse
TR (long)
180 o
pulse
TE (long)
Figure 3.46
39
MRI Physics & Instrumentation Notes
With long TR, there are no prevailing differences in T1. With long TE, however,
differences in T2 become pronounced.
To obtain spin-density weighting, we use a long TR and a short TE (as shown
in Figure 3.47.
short T1 (tissue 1)
T2 relax. (tissue 1)
long T1 (tissue 2)
spin echoes
90 o
pulse
TR (long)
T2 relax.
(tissue 2)
90 o
pulse
TE
(short)
Figure 3.47
By using a long TR and a short TE, neither T1- nor T2- weighting will occur*.
Instead, the image obtained will be spin-density weighted, with those tissues
possessing a greater proton density giving a larger spin-echo amplitude (and hence
a brighter image) than tissues with less spin-(proton-) density.
* Strictly speaking, the asterisked statement above is not quite correct. We must
be aware that any real image will always have some contribution from both T1
and T2 as well as spin-density. For example, in a T1-weighted image, although
TE is short, some T2 relaxation can and will occur in this period, and differences
in T2 between tissues will contribute and complicate the T1-weighted image.
Ideally to eliminate this, we must have TE = 0 which is not possible! Likewise,
with T2-weighted images, it is necessary to have TR so long that T1 contributions
are minimal. However, liquids such as CSF, urine, etc. have T1 of the order of
2-3 sec. (for the normal B field strengths used), so TR would have to be at least
~ 90 sec, which is unacceptably long in the imaging process. Therefore it is
difficult to avoid some T1-weighting in T2 images of tissues containing CSF,
urine, etc. For similar reasons it can be appreciated that spin-density weighted
images will inevitably be complicated by some T1- and T2- contrast.
We should also note that all images will possess some proton-(spin-) density
weighting, whether or not T1- or T2- weighting is superimposed. Different tissues
have different spin-density: the airways, lungs and intestinal gas contain very
little hydrogen nuclei (protons), and so have negligible magnetisation.
Consequently, they emit no appreciable signal, and always appear dark on
images. Cortical bone, other calcified structures and certain dense collagenous
tissues also appear dark on all MR images. This is not because they have no
hydrogen nuclei. Indeed they possess quite a lot of H-nuclei, but these nuclei are
40
MRI Physics & Instrumentation Notes
very tightly bound within their molecules, unlike the “mobile” H-nuclei found in
liquids and soft-tissue. This immobility of H-nuclei in bone prevents the
production of a detectable signal. Also, as we have seen earlier, because bone has
an extremely short T2 and a very long T1 (refer to Figure 3.29), there will be
negligible signal from bone on T1 and T2 weighted images. The immobility of
the H-nuclei will make bone also appear dark on spin-density weighted images.
Soft-tissues have intermediate spin densities, but these vary relatively little
between one tissue and another (so there is relatively little soft-tissue contrast on
spin-density weighted images). However some difference is noticeable, but what
is seen depends very much on the chosen TR and TE. CSF has high spin(proton-) density compared to the grey and white matter of the brain, and so
should look brighter on a true spin-density weighted image (with long TR and
very short TE).
41
MRI Physics & Instrumentation Notes
3
Echo sequences and image generation
3.1 The spin echo sequence
The spin echo (SE) sequence is a basic sequence used in clinical MRI. The
contrast weightings of T1, T2 and proton density found in SE are most wide
accepted weigthing of all sequences. A simple method to assist in understanding
contrast dependence of TR and TE is in the table below. Note these are contrast
weighted images. MRI cannot directly measure T1 and all spin echo images will
show both T1 and T2 within the image. By the use of these TR and TE
combination, weightings of the desired contrast can be achieved.
Long TE
Short TE
Long TR
T2 weighted
Proton Density
Short TR
Mixed wieghting
T1 weighted
Contrast dependence on TR and TE in Spin Echo Sequences
Figure 3.48A. T2 Weighted Image
Figure 3.48C. T1 Weighted Image
42
Figure 3.48B. Proton Density Weighted Image
MRI Physics & Instrumentation Notes
TE
TR (No.1)
TR (No.2)
tau
180°
90°
90°
RF
Signal
Figure 3.49
A Spin Echo sequence pulse train showing RF and signal components
Pulse Trains are used to examine individual events within all pulse sequences.
Figure 3.49 shows the first few lines of a SE pulse train, with lines to indicate the
timing of the RF pulses and the signal. Note the time between the 90 pulse and
the 180 is called tau. TE equals 2 times tau. Maximum signal intensity occurs at
exactly 2 times tau, TE, but there is signal received a few milliseconds before and
after the time TE.
TR is the time between consecutive 90 pulses. TR here is shown much longer
than TE, the reasons are discussed above.
3.2 Multiple echo sequences
Spin echo sequences can return more than one echo or signal. Often this is used to
obtain 2 images of 2 different contrast weightings from the same sequence. These
multiple signals are received within the one TR. For example, within a long TR,
say 2000 msec, a short and long TE can be used to collect signals that will be
proton density weighted and T2 weighted. This can be seen in the pulse train in
Figure 3.50.
90°
180°
180°
RF
Signal
PD weighting
T2 weighting
Figure 3.50
A Spin Echo sequence pulse train showing dual echoes
43
MRI Physics & Instrumentation Notes
It must be noted that TE2 can not be exactly double TE1. Signals cannot be
emitted and received at the same time. (Note: tau is half of TE so these would
occur at the same time. Also, gradients for slice selection and frequency encoding
must be turned on differing times. If the transmitted RF and received RF (signal)
occur at the same time, slice selection gradients and readout gradients would be
activated simultaneously. This will be discussed in detail later.
3.2 Multiple slice imaging
Slice 1
90°
RF
180°
90°
Signal
Slice 2
90°
180°
90°
RF
Signal
180°
Slice 3
RF
90°
Signal
Figure 3.51
A Spin Echo sequence pulse train showing multiple slice acquisition
Multiple slices can be collected within one sequence. If only one slice was to be
collected per sequence, the examination time would be very long (scan time is
discussed below). Between the signal collection and the next 90 pulse there is a
large amount of free time (see Figure 3.49). This free time can be utilised to
collect additional slices. Figure 3.51 shows the sequencing between various
slices. When the signal from slice 1 has been received, slice 2 is then able to be
initiated and its 90 and 180 pulses are able to be generated. The number of
slices that are able to be achieved per sequence is “very approximately equal to”
TR / TE. For example, with a TR of 2000 msec and a TE of 50msec, 40 slices
could be acquired. Obviously, slices 2’s 90 pulse cannot be initiated at exactly
TE, as the signal from slice 1 is still be collected for a few milliseconds after the
time of TE. This adds time to the denominator of the above equation. Also, other
factors such as gradient ramping times, etc will also add to this time and hence
reduce the number of slices. Generally, the longer the TR and the shorter the TE,
the more slices that can be gained during a sequence.
In summary, multiple slice imaging within one sequence utilises the ‘free space’
between the end signal being received and the beginning of the next 90 pulse or
TR period. Utilisation of this ‘free space’ allows multiple slices to be collected
and reduces the scanning time for the patient
44
90°
MRI Physics & Instrumentation Notes
3.3 Spatial localisation
Spatial localisation enables the MR signal to be converted into an image. Without
this localisation, MR spectroscopy would result. Localisation is needed in three
directions, i.e. Z direction and the X and Y directions.
Y
magnetic
field B
Z
X
Figure 3.52
As the above Figure 3.52 shows, Z direction is generally referred to be in the
same direction as B. MRI has a major advantage over other imaging modalities
such as CT, it can acquire two dimensional (2D) images in any plane, axial,
coronal sagittal and non-orthogonal planes. For the sake of simplicity, the
following discussion will assume an axial slice image, although the principles are
equally valid in any plane of image collection.
Spatial localisation consists of three separate areas:Slice Selection
Phase Encoding
Frequency Encoding
3.3.1 Slice selection
3.3.1.1 Z gradients
Gradient coils are used to vary the magnetic field strength in the Z direction, as
seen in 3.53. Current flows the gradients coils adds and / or subtracts from B
depending of the direction of the current flow. The gradients generally used only
change B by a few milliTesla. Figure 3.53 has be exaggerated for the sake of
simplicity.
Left
(foot end)
Right
(head end)
Z-gradient Coil
(over patient)
1.4
9
1.5
1.6
60
64
68
Tesla
MHz
Figure 3.53
Without the gradient variation of the magnetic field, the RF pulse, at the Larmor
frequency, will induce the Net Magnetisation Vector (NMV) to flip throughout the
45
MRI Physics & Instrumentation Notes
whole body. With the gradient turned on, only a thin section, i.e. the slice, will be
at 1.5T, and this will be the only section of the body to respond to the RF at the
Larmor frequency. Other sections of the body outside of this area will be at a
slightly differing magnetic field strength and hence require differing Lamor
frequency RF to flip the NMV.
3.3.1.2
Slice thickness
Slice thickness is controlled by two factors, gradient strength and RF bandwidth.
If the current through the gradient coils at each end is increased, the gradient
strength, that is the slope of the gradient, will increase. As the gradient becomes
steeper, less of the body will be at 1.5T and the slice will become thinner. This is
assuming a constant bandwidth of the RF pulse. The RF pulse that is transmitted
into the body is not just one frequency, it has a range of frequencies on either side
of the centre frequency of 63.87MHz for a 1.5T system. The broader the range,
i.e. bandwidth, of frequencies transmitted, the more of the body will be in a B that
has a Larmor frequency within the bandwidth. That is, the broader the bandwidth,
the thicker the slice. A combination of both gradient strength and bandwidth, as
seen in Figure 3.54, will control the slice thickness
gradient
A
(a)
60
bandwidth
64 65
68
MH z
68
MH z
72
MH z
{
slice 1
w
(b)
60
x
slice thickness
B
64 65
{
slice 2
(c)
gradient
- reduce strength
bandwidth
56
64 65
{
slice 3
Slice thickness
- increased
Figure 3.54
Diagram A in Figure 3.54, compared to Diagram B, using the same bandwidth,
shows varying slice thickness for differing gradient strengths (slopes). The result
of using a combination of gradient strength and bandwidth variations will be:If we use RF pulse with bandwidth 64-65 Mhz, we get slice thickness shown
in (a)
If the bandwidth of RF pulse is only 64-64.5 Mhz, get thinner slice as shown
in (b).
If pulse bandwidth is not altered, slice thickness can be altered by slope of
gradient field. A steeper gradient will give a thinner slice. (In Figure (c)
46
MRI Physics & Instrumentation Notes
have a steeper gradient across length of body same bandwidth as in
Figure (a) gives thinner slice).
3.3.1.3 Slice location
Slice location is controlled by one of two methods, depending upon the MR
system. The first method is to change the frequency, keeping the bandwidth the
same and maintaining the gradient in the Z direction. Each slice will be controlled
by the centre frequency of the RF pulse. The RF pulse’s Larmor frequency
relative to the gradient, i.e. the local B, will control where in the magnetic field the
NMV will respond to this RF pulse.
In the second method, the current that is passed through the gradient coils at each
end of the magnet bore is varied. This will control the relative strength of the
gradient at each end of the bore. As the current is lowered at the foot end of the
bore and increased in relative strength at the head end, the slice which is at 1.5T,
will move towards the patient’s feet. In this case, the RF pulse’s centre frequency
is kept the same, so the slice in which the NMV are responding effectively move
with the gradient.
3.3.1.4 Slice gradient timing
Timing of when the gradient for slice selection is turned on and off can be seen in
the Figure 3.55. Every time RF is transmitted into the patient it must be localised
by turning on Gz (sometimes shown as Gss). Both the 90 and the 180 pulses
time with the slice selection gradient.
90°
180°
90°
RF
Signal
Gz
Figure 3.55
A Spin Echo sequence pulse train showing slice selection gradient, Gz
3.3.1.5 Slice spacing
Slices are collected so there is a gap between individual slices, i.e. there is an
inter-slice gap (ISG). As is shown in Figure 3.56, the ideal slice profile, similar
to looking at the slice side on, is that of a rectangle. The real profile is that of a
gausian shape. If the slices are close together as in Figure 3.56A, there will be a
phenomena of cross-talk. This overlap of the slices means there are protons that
will be influenced by RF from 90 and 180 pulses from 2 slices. The
longitudinal magnetisation of the NMV in slice 1 will not have fully recovered
47
MRI Physics & Instrumentation Notes
before it receives some RF from slice 2. This means the NMV, in subsequent TRs
will saturate or end up below the XY plane which will cause degradation to slice
1. While this is occurring, slice 1’s RF will add signal to slice 2. This will again
cause saturation to NMV in slice 2. These two phenomena cause interference in
each slice.
Actual
slice
profile
A
Slice
1
Ideal
slice
profile
B
Slice
1
Slice
2
Slice
2
No
cross-talk
Area of crosstalk
Figure 3.56
In Figure 3.56B above, the ISG is large enough for the actual slice profiles not to
interfere with the NMV in the other slice. There will be no degradation from
cross-talk. Some typical slice thickness and ISGs are 5 / 2, i.e. 5mm slice with 2
mm ISG and 8 / 3, i.e. 8mm slice with 3 mm ISG.
3.3.2 X & Y gradients
Gradient coils, for control of the Z gradient, were shown above which increased
and decreased B at both ends of the magnet bore. Similarly X and Y gradient
coils are required to increase and decrease B in the X and Y directions.
Commonly, magnetic bores are cylindrical and circular coils used in the Z
direction would not fit into the bore in the X and Y directions. Instead the X and
Y coils are a modified circle to fit the bore, that is in the shape of a saddle as seen
in the Figure 3.57.
B
Gradient coils in Y direction
Figure 3.57
48
MRI Physics & Instrumentation Notes
3.3.3 Phase encoding
Following slice selection, the Z direction of the patient’s body has been localised.
Phase encoding is the first process which is used to localise the signal in the XY
plane.
Y
Y
90o pulse
Z
Z (B)
X
X
Figure 3.58
As shown in Figure 3.58, the NMV are aligned and precessing due to influence of
B. At point, no RF pulse has been emitted into the patient. Following the initial
90 pulse, with the associated Gz to localise the slice, the NMV are flipped into
the XY plane. These NMV, or now MXY, immediately beginning to precess in the
XY plane and start to dephase due to local magnetic inhomogeniety. If at this
time, a gradient, using the same principles as discussed above, is applied in the Y
direction, as seen below in Figure 3.59, a variation in B will exist along the Y
direction. According to the Larmor theorem, those MXY in the top row, where B is
greatest, will begin to precess faster than those in a B that is smaller. When the
gradient in the Y direction, Gy, is turned off a few milliseconds later, B will no
longer vary in the Y direction and all the MXY in the slice will resume precessing
at the same speed. Due to the varying precessional speeds during the time of Gy,
the MXY in the top row will have travelled further than those in the bottom row.
That is, the MXY in the top row will now be precessing at the same frequency but
at a different phase to the other rows.
Y
Y
Y
B+B
“top row”
+
Z
B
Z
“bottom row”
X
X
X
B-B
(Y-field gradient)
(on only briefly)
Vectors precessing in top row
lead middle row (by 90o) and
lead bottom row (by 180o in this
example)
Figure 3.59
49
MRI Physics & Instrumentation Notes
One specific phase of the signal that is received following the 90 and 180
pulses, is placed in an area in the computer known as K space. This one phase has
signal that corresponds to one row in the slice. To obtain signals that contain
information about all the rows of that slice, the process must be repeated until all
the rows required within the slice are collected. The number of phase encoding
steps varies depending upon the area of anatomy and spatial resolution required.
Typical figures 192 to 256. The pulse train diagram in Figure 3.60 below shows
the timing of Gy and indicated in TR no.2, a different phase of the signal is layed
down in K space.
180°
90°
90°
RF
Signal
Gz
Gy
Figure 3.60
A Spin Echo sequence pulse train showing phase encoding gradients, Gy
3.3.4 Frequency encoding
After the slice selection and phase encoding processes, the signal that is received
has characteristics that localise it in 2 dimensions. Localising the signal in the
third dimension is called Frequency encoding. This process again utilises gradient
field changes, but this time in the X direction. This is the Gx gradient, or
sometimes shown as Gfe or Gro for the read-out gradient. It utilises the same
principle as phase encoding, that is when NMV are in different B strengths, they
will precess at different speeds or frequencies. As seen in the Figure 3.61, each
column will have a unique precessional frequency.
Y
Y
Y
B-B
“right
column”
“right
column”
+
63
64
“left
column”
64
64
64
64
Z
65
B
64
Z
65
64
63
64
65 64
64 64
X
X
X
B+B
Figure 3.61
50
63
64
each voxel
characterized
by a unique
phase and frequency
combination
MRI Physics & Instrumentation Notes
The signal that is received, following the 90 and 180 pulses for each TR will
come from a specific volume or slice within the patient, have a unique phase
corresponding to a row and now will have varying frequencies which will identify
the columns. The timing of the frequency encoding gradient, Gx, can be seen in
Figure 3.62. Gx is turned on when the signal is to be received. This is often
called the readout gradient and occurs for every TR when receiving the signal.
TR
180°
TE
90°
90°
RF
Signal
Gz
Gy
Gx
Figure 3.62
A Spin Echo sequence pulse train showing frequency encoding gradient, Gx
3.3.5 Spatial Encoding Pulse Train
A review of the spatial localisation process can be achieved by examining the
pulse train in Figure 3.62. This sequence can divided into five time segments per
TR. Following the first TR, the sequence is repeated in TR no. 2. Looking at
each segment:
1.
Slice-selection - achieved through appropriate choice and application of Gz
immediately prior to and during 90 RF pulse. Duration of Gz ~ 5 ms.
2.
Phase-encoding gradient Gy, is applied immediately after the 90 pulse and
Gz pulse. This gradient increases precessional speed of the MXY vectors in
consecutive “rows” within tissue slice.. The gradient is switched off within
about 5 ms and the MXY will return to the same precessional speed but be a
fixed amount out of phase.
3.
Echo preparation time - immediately after Gy, is turned off, the MXY vectors
throughout the tissue slice will all precess about applied field B at the same
(Larmor) frequency, wo = B, but every “row” of vectors will now be a fixed
amount out of phase with neighbouring rows. As time progresses, the
51
MRI Physics & Instrumentation Notes
precessing MXY start to decay in magnitude (due to T2* dephasing of
precessing proton dipole moments). So it is necessary to rephase these
dipole moments by applying a 180 pulse. (Note that this dipole rephasing
(which produces the spin-echo) and 180 pulse will not alter the “frozen-in”
phase differences between MXY in different “rows” due to Gy, since (a) the
MXY vectors continue to precess at Larmor frequency (they merely decay
with time) and (b) the 180 pulse merely rotates every MXY vector in the
slice by exactly the same amount).
In order that the applied 180 pulse only affect magnetisations in the
selected slice and not disturb neighbouring tissue volume, it is necessary to
switch on Gz again immediately prior to, and during this 180 pulse.
The exact time of application of the 180 pulse is governed by whether a
short or long TE value is desired.
4.
Echo detection - As soon as the 180 pulse is completed, the MXY vectors in
every voxel start to re-grow (due to dipole rephasing). Immediately prior to
the time to receive the signal, the frequency encoding gradient, Gx is
switched on. This gradient starts the MXY vectors in different “columns” of
the slice precessing at different frequencies. Consequently, the net spinecho emission produced will be a mix of signals with different RF
frequencies as well as different phases and amplitudes. Note that maximum
signal amplitude occurs at exactly the time of TE when re-phasing is the
greatest. Slightly before and after the time of TE the re-phasing is not fully
complete or starting to dephase again, hence the NMV is reduce and the
amplitude of the signal is less.
5.
Following spin-echo detection, Gx is switched off. This leaves a variable
time interval before a new sequence is commenced. This time interval is
governed by the desired TR for the sequence, and can vary from between
400 and 4000 ms. This relatively long time period is not allowed to idly
pass, but is profitably used to commence imaging of other adjacent slices of
tissue, referred to as multislice imaging (discussed above).
6.
At the end of the TR interval, a new sequence commences. The sliceselection gradient Gz used in the first sequence, and the 90 pulse, are reapplied, but for this TR, the signal that is laid down in K space has a
difference phase from the previous TR interval. The gradient Gy is the same
strength and location but is represented by GY2 to indicate a signal of
different phase.
3.4 Scan time
A phase encoding step is required for every row within the slice. For example if a
spatial resolution of 256 is required in the phase encoding direction, 256 TR’s will
have to be performed to divide the slice into 256 rows. Scan time then is
dependant upon the length of the TR and the number of phase encoding steps. A
52
MRI Physics & Instrumentation Notes
third contributing factor is the number of times the scan is repeated. This is
known as the number of acquisitions or excitations, called NEX. This is done to
increase the signal to noise ratio (SNR), hence improving the quality of the
images.
SNR NEX, for example to double the SNR for a scan, the NEX would need to
increased by 4 times.
Scan time then is a factor of these three event and is:Scan time = TR x PE steps x NEX
Whereas with phase encoding, for every row required there needs to PE step,
frequency encoding occurs within each TR interval. To increase the spatial
resolution in the frequency encoding direction the frequencies from the signal are
broken down into smaller steps. This does not require an increase the scan time.
There is a saying in MRI to reflect this, that is “frequency encoding costs
nothing”. It is common to see the spatial resolution much greater, for example
512, in the frequency encoding direction than in the phase encoding direction.
3.5 Other pulse sequences
The spin echo sequence is the basic pulse sequence. There are many other
sequences which are used in clinical MR imaging. This section is not designed to
give the concepts of such sequences but it is worth listing the types of sequences
and some advantages and disadvantages of these compared to the SE sequence.
Inversion Recovery (IR)
Heavily T1 weighted image. – very useful for paediatric neurological scans
Similar in scan time to SE T2 weighted images (a long scan time)
Subsequences of IR are:Short TI Inversion Recovery (STIR)
(note TI is the time of inversion and is not T1)
suppresses the fat signal in the image
Fluid Attenuated Inversion Recovery (FLAIR)
suppresses water signal in the image
Fast Spin Echo (FSE) or Turbo Spin Echo
Very similar contrast (T1, T2 and PD) to conventional SE.
Very much reduced scan times over conventional SE.
Reduction of scan time is often traded off for:increased T2 weighting
increased SNR, i.e. increase the no. of NEX
increase spatial resolution, i.e. increase the no. of phase encoding
steps.
53
MRI Physics & Instrumentation Notes
Gradient Echo
Contrast weighting differs from SE and can cause some confusion when
viewing the images. T2* is the main image contrast with T1 and even
T2 possible.
Useful in showing magnetic susceptibility, which can be used to age
intracranial bleeds.
Uses gradients to refocus the dephasing MXY vectors (instead of 180 RF
pulses) and generally has flip angles less than 90
Very short scan times due to short flip angles and not having to wait for T2
dephasing.
3D Imaging
The above sequences, including the major discussion on Spin Echo, have
been two dimensional (2D) imaging. Although the slice has a defined
thickness, the signal is reconstructed to show a 2D image. It is
possible to acquire a larger volume of the body, say 50mm, as per 2D
imaging, and include additional phase encoding steps in the Z
direction, to subslice this large 50mm volume.
Advantages of this is, slices can become:- very thin, e.g. 1mm with out a large loss of signal
- can have no interslice gap (ISG) without the problem of cross-talk
of 2D imaging
The disadvantage of 3D acquisitions is the scan time.
Scan time = TR x PE steps x NEX x Slice encoding steps
A 3D acquisition may require 10 slices, hence this acquisition will be
10 times longer than its 2D counterpart (with the same TR, PE steps
and NEX).
3D imaging, due to this long time, is normally done using gradient echo
imaging where a typical TR is 50msec compared to a TR in SE of
2000msec.
3.6 Safety in MRI
Although there is no ionising radiation in MRI, there are real dangers. Operators
must be aware of these dangers and ensure patients and staff conform to the safety
procedures before entering the scan room. MRI safety falls into 3 major areas:- magnetic field , static and time varying
- RF exposure
- Cryostat
3.6.1 Static magnet field
The static magnetic field is that magnetic field, without any time variation or
objects or patients moving through the main magnetic filed.
54
MRI Physics & Instrumentation Notes
Biological effects
There is no data to suggest danger of static field effects to;
- ECG - (permanent)
- carcinogenesis
- leukaemia
Pregnancy
- no known biological effects
- suggested that any MRI examine be delay until after the 1st trimester, due
to most foetal development occurs in 1st trimester
- contrast (gadolinium) avoided during pregnancy
In magnetic fields above 2T
ECG
- shown increase in T wave
- conductive fluid eg: blood moves across a mag field
Body Temp
- slight increase in body temp (0.03-0.1C) when exposed to B for 20 +
mins.
Projectiles
The main danger in a static magnetic field is that of projectiles.
Ferromagnetic objects are strong attracted to B’s of such strength and the
object will rapidly accelerate to towards the centre of the gantry.
- any ferromagnetic material must be excluded from the scan room ie: 0.5
mT fringe field.
- non ferrous medical equipment must be used in scan room
eg: 02 tanks, drip stands, anaesthetic units, etc
- The patient and ancillary staff must be fully informed of these dangers and
be screened prior to entry and ferromagnetic objects not be allowed in the
scan room, Such objects as hair pins, scissors, pocket knives etc may be
ferromagnetic
- Patients with metallic foreign bodies, especially in eye, must be treated
with extreme caution and possibly exclude from MRI examinations.
Most MRI sites have policies that metal workers may require x-ray prior
to MRI scan to ensure no intraoccular foreign body.
- Watches, especially analogue watches, are affected by a static magnetic
field and may cause the watch to stop functioning.
3.6.2 Time varying magnetic fields
Time varying magnetic fields are when the magnetic field is varying over a time
period. This includes the use of gradients and moving objects or patients across
static magnetic fields.
55
MRI Physics & Instrumentation Notes
Torque and Heating
When metallic objects are subjected to time varying magnetic fields, they
exhibit torque (twisting) and exhibit an increase in temperature
Patients must be excluded from MRI scans if they have:
- metallic implant/prosthesis
- aneurism clips
- vascular clips
- intravascular filters/stents
- heart valves
- surgical implants/clips
- other devices also can be deflected
many of these devices are now MRI compatible and compatibility lists
available and are regularly updated. Stringent tests within a variety of
magnetic field strengths must be conducted to have devices listed.
Electrical Induction
Currents can be induced in conductive devices such as wires or leads. This
induction of current can interrupt the normal operation of the device,
especially if sensitive electronics are an integral part of the device.
Patients with:- cardiac pacemakers MUST be excluded from 0.5m T fringe filed
line
- electrical activated devices
- cochlear implants
- neurostimulators
- drug infusion pumps
must also be excluded from MRI examinations
3.6.3 RF exposure
Specific absorption rate (SAR) is a measure of the RF energy dissipation and
measured in watts/kg.
- the main factors affecting SAR are:
- main magnetic field strength. The higher B, the higher the amplitude /
power of RF (as well as the frequency) that is needed to flip the NMV
- patient weight and tissue density
The Federal Drug and Food Administration (FDA) in the USA
recommendation maximum figures for SAR of:
- whole body - 0.4 w/kg
- head - 3.2 w/kg
- small volumes - 8 w/kg
These are currently under review and there may be recommendations
limiting RF deposition so that body temperature does not increase more
than 1.8C
56
MRI Physics & Instrumentation Notes
Scanning influences on the amount of RF deposited within the patient are:
flip angle, the larger this angle, the more RF required
gradient echo imaging uses les RF than spin echo which uses less
RF than fast spin echo imaging
use of special image improvement techniques such as saturation
pulses, saturation bands, chemical saturation, contrast prepared
pulses.
3.6.4 Cryostat
Cryostat is a liquid gas, such as helium, which maintain the superconductivity of
the coils which provide the current to induce the main magnet field.
An uncontrolled quench is loss of superconductivity in uncontrolled manner in the
magnet. The loss of superconductivity increases resistance in main magnetic
coils, which inturn increase heat within the coils, which inturn increase the boil
off rate of the liquid helium which reduces superconductivity. This cycle becomes
very rapid and He gas is rapidly produced. The He gas has potential to rapidly
force the air out of the scan room potential suffocating patients and staff in the
room.
Needed in the scan room to monitor this potential situation is an oxygen alarm.
This should have a dual function of raising the alarm to staff and importantly,
activating large exhaust ducts to start extraction of the He gas.
Controlled quences may be used in an emergency such as when a large
ferromagnetic object has a patient trapped. This gradually boils off the liquid
helium to stop the magnet. Either type of quence will potentially damage the
magnet worth hundreds of thousand of dollars, and at least require the magent to
be refilled with liquid helium which would cost approximately $100,000 to refill.
57
MRI Physics & Instrumentation Notes
4
Self-assessment questions
1.
Outline two significant advantages of MRI over CT in soft-tissue imaging.
2.
A sample of helium nuclei (with each nucleus containing 2 protons and 2
neutrons) is placed in a magnetic field.
a. Is the nuclear spin quantum number, I
i. an odd multiple of (1/2)
ii. zero
iii. an integer
b.
Is it possible to obtain an MR image of this sample? Explain your
answer.
3.
Explain how and why a nuclear magnetic dipole moment, is different in
behaviour to a bar-magnet or compass needle in a magnetic field.
4.
Calculate the ratio of the energies of a 120 keV X-ray beam and a 50 MHz
radiofrequency (RF) pulse. What conclusion/statement does your result allow
you to make? Take the electron charge, e = 1.6 x 10-19 C and Planck’s
Constant, h = 6.63 x 10-34Js.
5.
A sample of CSF has 8 x 1016 excess spin-up dipoles in an applied B field.
How many spin-up dipoles would remain immediately following a 90 RF
pulse ? Explain the reasoning behind your answer.
58
MRI Physics & Instrumentation Notes
6.
Briefly explain and distinguish between the physical mechanisms associated
with (a) Transverse (spin-spin) and (b) Longitudinal (spin-lattice) relaxation
in MRI.
7.
Distinguish between T2 and T2 relaxation.
8.
What do you understand by the “ FID” signal, and how it is detected ?
9.
What is “spin density” and what effect does this have the size of the
Magnetisation vector and on the FID signal ?
10. With the aid of a labelled diagram, briefly describe a simple spin-echo
sequence (90 - 180 - 180 - 180 ....), and explain what physical nuclear process
is involved in the formation of the spin-echo.
11. Explain why (i) bowel gas, and (ii) cortical bone always appear dark on MR
images.
59
MRI Physics & Instrumentation Notes
12. Discuss the importance of the parameters, TE and TR in MRI.
13. Discuss the parameter variations of the spin echo sequences which give rise to
T1, T2 and PD weighted images.
14. Using a pulse train diagram, explain the timing of the gradients with RF
transmitted and received signals.
15. Explain how slice thickness is controlled in MRI.
16. Discuss the steps required to spatially localise the signal in a 2D MR image.
17. Briefly list and discuss advantages and disadvantages of the major pulse
sequence used in MRI.
18. What safety precautions must be taken for the patient prior to having a MRI
scan?
60
MRI Physics & Instrumentation Notes
5
Recommended references
Hashemi, R.H. and Bradley, W.G. (1997). MRI: The basics. Williams and
Wilkins: Baltimore.
Newhouse, J.H. and Wiener, J.I. (1991). Understanding MRI. Little, Brown &
Co.
Curry, T.S., Dowdey, J.E., and Murray, R.C. (1990). Christensen’s introduction
to the physics of diagnostic radiology, 4th ed., Lea & Febiger: Philadelphia.
Bushburg, J.T., Seibert, J.A., Leidholdt, E.M. and Boone, J.M. (1994). The
essential physics of medical imaging. Williams and Wilkins: Baltimore
Bushong, S.C. (1996). Magnetic resonance imaging: Physical and biological
principles, 2nd Ed., Mosby: St Louis.
Westbrook, C. and Kaut, C. (1993). MRI in practice. Blackwell Science: Oxford
Woodward, P. and Freimarch, R. (1995) MRI for Technologists, McGraw Hill,
New York
Schild, H.H. (1990). MRI made easy (... Well almost). Schering.
61