Brandon Gustafson Sustainable Air Quality
advertisement

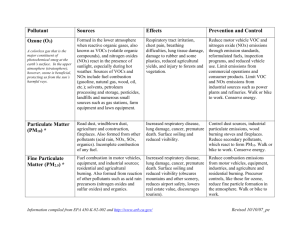
Brandon Gustafson Sustainable Air Quality- EPAAQ Summary 2001 As described in the EPA report on EPAAQ trends the six major pollutants are: Nitrogen Dioxide (NO2) Ozone (O3) Sulfur Dioxide (SO2) Particulate Matter (PM) Carbon Monoxide (CO) Lead (Pb). All of these pollutants are regulated under the federal Clean Air Act which gives responsibilities to the EPA to attempt to regulate and control these harmful pollutants. Most of these pollutants are directly emitted; however, Ozone is a secondary pollutant that is a combination of volatile organics compounds (VOC) and Nitrogen Dioxide that react in the presence of sunlight. Particulate matter can also be produced indirectly as a secondary pollutant but is also produced directly. Each pollutant is a little different and is discussed in the following paragraphs. Nitrogen Dioxide (NO2) Nitrogen Dioxide (NO2) is a reddish brown gas that is highly reactive. It is formed in ambient air through the oxidation of nitric oxide (NO). The general term for nitrogen oxides is (NOx) and refers to the sum of a number of nitrogen oxides. These pollutants play a major role in ozone formation, particulate matter formation, haze and acid rain. Major Sources The major sources of NOx emissions are high-temperature combustion processes. Typically these occur in automobiles and power plants but also home heaters and gas stoves. These latter two sources can produce substantial amounts of pollution indoors. Major Effects The major effects to humans from this type of pollution are broken down into two categories: short-term exposure and long-term exposure. Short-term exposure, usually thought of as less than 3 hours, at even low level of nitrogen dioxide can lead to changes in airway responsiveness and lunch function in those individuals with preexisting respiratory illness. This same exposure can cause respiratory illness in otherwise healthy children. Long-term exposure to this type of pollution can lead to increased susceptibility to respiratory infection and may cause irreversible alterations in lung structure. NOx particles react in the ambient air to form ground-level ozone and fine particle pollution. Both of these situations are tied to adverse health effects. Besides direct effects to humans NOx pollutants contribute to other environmental effects as well. Either directly or indirectly by combining with other pollutants, NOx is a Brandon Gustafson precursor for acid rain and ozone. The excess nitrogen inputted into terrestrial and wetland systems can have an extended effect on existing competitive relationships among plant species that lead to changes in composition and diversity within the plant community. In addition, the direct input of nitrogen to aquatic systems can lead to eutrophication, a condition which can lead to excessive algae growth and a severe depletion of dissolved oxygen. Nitrogen along or through acid rain acts to acidify the solids and waters it touches. The increase in acid concentration causes the plants to loose essential nutrients. The increased acidity also increases the levels of soluble aluminum which is toxic to plants. The low pH created and increased levels of aluminum are toxic to fish and other aquatic organisms. Besides these harmful effects, NOx also contributes to visibility impairment. Spatial Distribution The main areas of concentration are around cities. This is because cities contain the major sources of this type of pollution. Big cities on either coast and in the south contain this type of pollutant. Although all major cities experience this type of pollution. Time Trend In terms of trends the measured pollutant nitrogen dioxide as decreased 24 percent over the last 20 years. All areas of the country that once violated the NAAQS for this pollutant now meet that standard. No area of the country currently suffers nitrogen dioxide levels above what the NAAQS calls for. Even though the air quality has improved the total emissions of NOx has actually increased by 9 percent. This is still a concern because of the effects mentioned earlier. Ozone (O3) Ozone is not emitted directly to the air but is a secondary pollutant. VOC’s and NOx combine in the presence of heat and sunlight to form ozone. This is especially prevalent in the hot summer months. Major Sources VOC’s are emitted from a lot of different sources. The list includes motor vehicles, chemical plans, refineries, factories, consumer and commercial products and other industrial sources. NOx as previously described is emitted primarily from sources of combustion. Ozone with the help of wind can be spread hundreds of miles. Major Effects Ozone exposure has been tied to health effects including decreased lung function and increased respiratory symptoms such as chest pain and a cough. Exposure to ozone makes people more susceptible to respiratory infection. It can result in lung inflammation and has the ability to aggravate preexisting respiratory diseases such as asthma. When the ozone levels rise there has been noticed an increase in hospital admissions for respiratory problems. These effects generally occur more often to people who are active outdoors during the high ozone times, like summer. Long term exposure Brandon Gustafson leaves the possibility of irreversible changes in lung structure and could lead to premature aging of the lungs. Ozone also affects agriculture and plant life, leading to lower yields. Spatial Distribution Ozone distribution is largest on the east coast and in the south. The southern west coast is similar with the least ozone being found in the Midwest-west regions. It is also more abundant around big cities as these are the places that produce the pollutants that form ozone. Time Trend Over the last 20 years the national ambient ozone levels have decreased 11 percent. This is do largely to a 16 percent reduction in VOC’s emissions but counteracted by an increase of 9 percent of NOx emissions. All areas of the country have improved although some areas more quickly than others. Sulfur Dioxide (SO2) Sulfur Dioxide is a gas that is formed when fuel containing sulfur is burned. Major Sources The major sources of sulfur dioxide are coal and oil. These fuels are burned during metal smelting and other industrial processes. Fuel combustion largely from coalfired plans accounts for much of the SO2 emissions. Major Effects Sulfur dioxide can cause breathing impairment for asthmatic individuals who are active outdoors. Symptoms of breathing difficulties include wheezing, chest tightness and shortness of breath. High levels of SO2 also are linked to aggravation of existing cardiovascular disease and respiratory illness. Sulfur dioxide is also a major precursor to acid rain. Discussed earlier with NOx emissions the acid rain is harmful to many ecosystems. These emissions also contribute to poor visibility. Spatial Distribution Sulfur dioxide is largely concentrated around the big cities that employ the power plants. The coal and oil fired combustion sources found in the east coast have the highest distribution. Any of the large populated areas contain sulfur dioxide pollutants due to the demand for energy. Time Trend Over the same time period SO2 emissions have decreased by 53 percent. These are thought to be due in large part to the EPA’s Acid Rain Program started in 1995. Particulate Matter (PM) Brandon Gustafson Particulate matter is a term that encompasses a mixture of solid particles and liquid droplets found in the air. They are divided into two groups. Those smaller than 2.5 micrometers and those between 10 and 2.5 micrometers. Major Sources PM can be directly emitted to the air as a primary particle or it can from in the air as a secondary particle. Examples of primary emitted particles are dust and elemental carbon. Secondary particles are formed from other pollutants like so2 and are usually emitted from power plants and other commercial activities. Major Effects Any particle that is small enough to get into the lung can cause problems and this is what any of these PM particles can do. Various health problems can arise that are similar to the other pollutants. They aggravate respiratory conditions like asthma and bronchitis. They have also been associated with heartbeat irregularities. As well as affecting health PM also contributes to poor visibility. Spatial Distribution The largest concentration of PM pollution occurs in southern California followed by the east coast as a general region. The mid-west to west does not have nearly the same levels of concentrations that the areas around the biggest cities do. Time Trend Over the last 10 years average PM10 concentrations are down 14 percent while direct emissions are down 13 percent. During this same time PM2.5 has also decreased by 10 percent. Carbon Monoxide (CO) Carbon monoxide is a colorless, odorless gas that is formed from incomplete combustion. Major Sources The most abundant source of the pollutant is motor vehicles. They account for 60 percent nation wide of CO emissions. Non-road vehicles account for the rest. High concentrations generally occur around traffic zones. But, other sources include industrial processes, non-transportation fuel combustion and natural sources like wild fires. Major Effects Carbon monoxide enters the bloodstream thought the lungs and directly reduces the oxygen the body can deliver to the orgasm and tissue. This is most serious for people who suffer from cardiovascular disease and higher levels can even be poisonous for a healthy individual. Spatial Distribution Brandon Gustafson Carbon monoxide as described earlier is contained around large traffic zones and areas that have a lot of combustion reactions taking place. Concentrations are higher around cities. Time Trend In the past 20 years CO levels have dropped 62 percent and are at the lowest levels in 20 years. The major contributor to CO, cars, has decreased emission slightly during this period. The air quality improvement came despite a 35 percent increase in vehicle miles driven. CO emissions are up 6 percent but this is due to an extremely serious wildfire season in 2000. Lead (Pb) Lead is natural element that is toxic to humans Major Sources Major sources used to include automobiles but due to the efforts to reduce lead in gasoline these types of emissions are greatly reduced. Today the primary culprits are industrial processes, primarily metals processing. The highest concentrations are found near smelters and battery manufacturers. Major Effects Lead, once in the body, accumulates in the blood, bones and soft tissues and can adversely affect the kidneys, liver and nervous system. Even in small doses lead exposure is linked to nervous system damage, lowered IQ and learning difficulties. Spatial Distribution Lead concentrations are very low almost everywhere but have there peaks near where leaded cars once drove. Off of highways was once a problem spot. Now lead is mostly concentrated around the industrial areas that emit the pollutant. Time Trend Due largely to the phase out of leaded gasoline lead concentrations are down 94 percent over the last 20 years while emissions are down 93 percent.